News reports continue to raise awareness of potential health threats associated with exposure to hazardous materials. The Elk River chemical spill in January 2014 caused deprivation of tap water to 300,000 people for weeks. Elsewhere, disturbing governmental reports on soil pollution advocate for more discernment in protecting our resources. As the EPA updates its screening program for endocrine disruptors such as phthalates, steroidal hormones, and bisphenol A (BPA), the effects of these molecules on biological functions are deeply scrutinized.
Together, these examples highlight the need for new approaches to prevent industrial discharges of chemicals and enable more efficient environmental remediation. In an article recently published in Nature Communications, researchers at the Massachusetts Institute of Technology (MIT) showed a proof-of-concept for a technology that could theoretically be developed for all of these applications. The technology, relying on the non-specific absorption of chemicals on the surface of nanoparticles, could enable efficient decontamination of both water and soils. The biodegradable nanoparticles exploit the high surface-to-volume ratio intrinsic to their small diameter and can be easily removed after use through photoinduced precipitation. This irremediable switching from nano- to macroscale allows limiting the environmental exposure to nanomaterials, possibly presenting a valuable argument toward a broader acceptance of the technology by regulatory agencies and the general public.
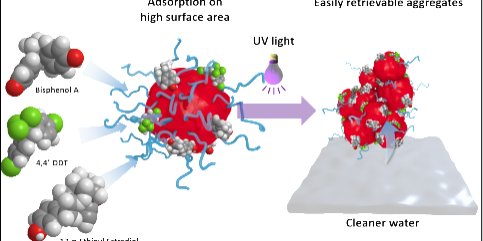
The nanoparticles are prepared from poly (ethylene glycol)-b-poly(lactic acid) (PEG-b-PLA) copolymers which are widely used for tissue engineering and drug delivery applications. To enable photoinduced switching from nano- to macroscale, a photolabile linker is introduced between the hydrophilic (PEG) and hydrophobic (PLA) block. By self-assembly in water, these copolymers form nanoparticles with a hydrophobic core and a hydrophilic stabilizing corona. On irradiation with ultraviolet (UV) light, the photolabile linker is cleaved and the protecting corona is shed. This destabilizes the nanoparticles and forces their aggregation in retrievable macroscopic solids that can be easily removed from water. Using that process, more than 95 percent of the nanoparticles can be recovered.
Hydrophobic interactions of chemicals with the nanoparticle surface, followed by removal of the nanomaterial from water allow mild, solvent-free extraction of different molecules. In this work, Drs. Ferdinand Brandl and Nicolas Bertrand, former postdoctoral researchers in the laboratory of Prof. Robert Langer at MIT, have tested their system on more than 22 different organic chemicals including endocrine disruptors, pesticides, and pharmaceutical compounds. They show that interactions between the nanomaterial and pollutants depend on the specific surface area of the nanoparticles and the inherent hydrophobicity of the chemicals. The system proved to be efficient in the removal of various biopersistent molecules including chlorinated pesticides (e.g., 4,4’-DDT), hormones (e.g., estradiol), plasticizers (e.g., phthalates), and polycyclic aromatic hydrocarbons. Since the photolabile linker reacts with UV light, the authors believe chemical removal occurs not only through the aforementioned extraction but also through photodegradation. They believe the combination of both principles allows increased performance and versatility while ensuring that no potentially harmful byproducts are left in the water.
Teratogenicity studies were conducted to feature that aspect and to highlight that the technology could be used to mitigate the developmental toxicity of various chemicals. The development of zebrafish embryos exposed to water containing harmful concentrations of pollutants but
treated with nanoparticles was not different from that observed in control groups (regular aquarium water). Finally, the feasibility of the approach was confirmed in three small-scale pilot studies on wastewater, thermal printing paper (an important industrial source of BPA), and contaminated soil. In all these experiments, nanoparticles proved to be suitable to effectively remove pollutants.
Although potential industrial applications of this technology are far down the road, the proof-of-concept offers a new perspective on addressing crucial environmental problems. Exploiting the properties of nanoparticles without exposing the environment to nanomaterials seems to
be particularly beneficial in these applications. Similarly, the utilization of UV light is also potentially interesting because UV irradiation is already a part of most water purification infrastructures. The article in Nature is available online at: http://www.nature.com/ncomms/2015/ 150721/ncomms8765/full/ncomms8765.html