Traumatic hemorrhaging is the leading cause of death on the battlefield, and the fifth leading cause of death in the U.S. [1, 2]. In contrast to lacerations and extremity injuries, deep abdominal wounds do not readily clot, are often incompressible, and cannot be sealed with tourniquets, bandages, or tissue adhesives.
These injuries require prompt surgical intervention to mitigate hemorrhaging and reduce the risk of morbidity caused by exsanguination [3]. Unfortunately, injured warfighters often have limited access to immediate surgical attention, and uncontrollable blood loss while in transit to a trauma center could potentially prove fatal [3, 4].
The Wound Stasis Systems Program
To address this problem, the Defense Advanced Research Projects Agency (DARPA) launched the Wound Stasis Systems (WSS) program in 2010. WSS was created to facilitate the development of novel biostasis treatment strategies to improve the survivability of traumatic injuries during medical transport [5]. One of the program’s primary goals was to identify a suitable material that could be safely applied to the abdominal cavity, bind damaged tissue, and conform to the intricate structures of internal organs. These efforts were highly successful, and in 2013, researchers disclosed the development of an expandable foam with unique hemostatic properties to help advance DARPA’s WSS program [6].
Expandable Foams
The expandable foam developed for DARPA’s WSS program consists of two polyurethane precursors that are delivered into the abdominal cavity via a pneumatically driven mixing gun [6]. The precursors rapidly polymerize on contact with air and dramatically expand to several times their initial volume to produce a rigid matrix that molds around vital organs. The foam stays in place for several hours and is removed from the abdominal cavity when the patient arrives on the operating table. This process is illustrated in Figure 1 as follows: (left) injury occurs and ResQFoam is deployed; (middle) ResQFoam forms in situ; and (right) ResQFoam is surgically extracted.
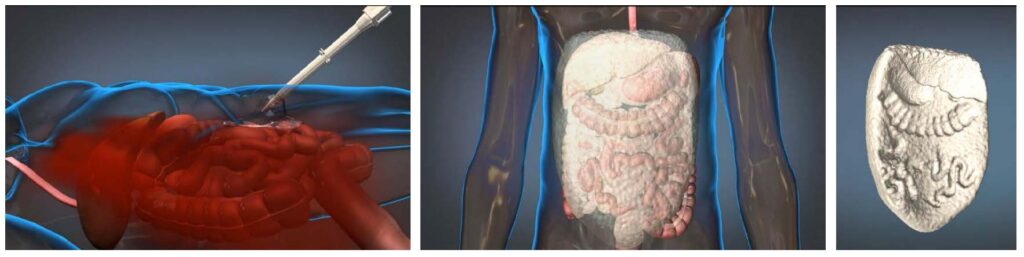
Figure 1. Overview of how ResQFoamTM expandable foam interacts with the body (Source: FDA)
Preliminary experiments quantified the survival rates of severe liver injuries of noncoagulopathic swine with and without foam treatment [6, 7]. In the control group (n = 12) the hepatic injury was found to be 90 percent fatal in less than an hour, and 1.5 L of blood was lost in the first 10 minutes. In the foam group (n = 15), 100 percent of the animals survived after one hour, and 73 percent survived after three hours [6].
It’s worth noting that the expanding foam overcame many hurdles, such as hydrodynamic forces associated with bleeding, complex anatomic topography, and the extensive pooling of blood in the abdominal cavity. Based on the success of these experiments, the clinical implications of expandable foams became immediately obvious, and the project transitioned to the U.S Army in 2015 for further development.
Subsequent studies were initiated to design a portable delivery device and assess the foam’s performance in various environments pertinent to military personnel. It was found that the foaming reaction occurred over a relatively broad temperature range (50-122 degrees Fahrenheit), with no significant difference in performance [8].
The precursors also performed well under accelerated aging conditions (heating at 122 degrees Fahrenheit for two months) to simulate one year at room temperature. Additionally, the foam did not produce organ dysfunction or toxicity in swine at either 28 or 90 days (the length of the study) after removal [9].
In collaboration with the U.S. Army, Arsenal Medical branded the technology as ResQFoamTM and initiated clinical trials in December 2018. The trials will evaluate efficacy and safety in human subjects (clinical trial number NCT02880163). Results from this study are eagerly anticipated.
Conclusion
Uncontrollable blood loss poses a significant threat to injured warfighters on the battlefield. Expandable foam may significantly increase survival time for injured soldiers who require immediate surgery. If proven efficacious, this technology has the potential to revolutionize trauma care in both civilian and military sectors.
REFERENCES
1. Eastridge, B., Hardin, M., Cantrell, J., Oetjen-Gerdes, L., Zubko, T., & Mallak, C., … & Blacknourne, L. (2011). Died of Wounds on the Battlefield: Causation and Implications for Improving Combat Casualty Care. The Journal Of Trauma: Injury, Infection, And Critical Care, 71(supplement), S4-S8. doi: 10.1097/ta.0b013e318221147b
2. Holcomb, J. (2004). Methods for improved hemorrhage control. Critical Care, 8(Suppl 2), S57. doi: 10.1186/cc2407
3. Martin, M., Oh, J., Currier, H., Tai, N., Beekley, A., Eckert, M., & Holcomb, J. (2009). An Analysis of In-Hospital Deaths at a Modern Combat Support Hospital. The Journal Of Trauma: Injury, Infection, And Critical Care, 66(Supplement), S51-S61. doi: 10.1097/ta.0b013e31819d86ad
4. Holcomb, J., McMullin, N., Pearse, L., Caruso, J., Wade, C., & Oetjen-Gerdes, L., … & Butler, F. (2007). Causes of Death in U.S. Special Operations Forces in the Global War on Terrorism. Annals Of Surgery, 245(6), 986-991. doi:10.1097/01.sla.0000259433.03754.98
5. Hepburn, M. Wound Stasis System (WSS). Retrieved from https://www.darpa.mil/program/wound-stasis-system
6. Duggan, M., Rago, A., Sharma, U., Zugates, G., Freyman, T., & Busold, R.,… & King, L. (2013). Self-expanding polyurethane polymer improves survival in a model of noncompressible massive abdominal hemorrhage. Journal Of Trauma And Acute Care Surgery, 74(6), 1462-1467. doi: 10.1097/ta.0b013e31828da937
7. Duggan, M., Mejaddam, A., Beagle, J., deMoya, M., Velmahosa, G., & Alam, H., … & King, D. (2013). Development of a lethal, closed-abdomen grade V hepato-portal injury model in non-coagulopathic swine. Journal Of Surgical Research, 182(1), 101-107. doi: 10.1016/j.jss.2012.07. 048
8. Rago, A., Larentzakis, A., Marini, J., Picard, A., Duggan, M., & Busold, R., … & King, D. (2015). Efficacy of a prehospital self-expanding polyurethane foam for noncompressible hemorrhage under extreme operational conditions. Journal Of Trauma And Acute Care Surgery, 78(2), 324-329. doi: 10.1097/ta.0000000000000507
9. Rago, A., Duggan, M., Hannett, P., Brennecke, L., LaRochelle, A., & Khatri, C., … & King, D. (2015). Chronic safety assessment of hemostatic self-expanding foam. Journal Of Trauma And Acute Care Surgery, 79, S78-S84. doi:10.1097/ta.0000000000000571


