Military medicine spans the gamut from emergency field care to rehabilitation. Across this spectrum technologies that are robust, portable and adaptive are critical for the patient’s recovery. Traditionally, medical robotics is associated with intricate, bulky and specialized equipment, which is deployed in major hospitals but is not relevant in the field. Micro-robotics, by contrast, advertises treatment delivery capabilities that are portable, robust and targeted. Recent progress in smart materials and fabrication techniques enables medical robots to be smaller, softer, smarter and safer than ever before. [1] Soft micro-robotics provides a tactical advantage for precision medical treatment in austere environments. Furthermore, the use of soft and smart polymers, such as hydrogels, eliminates many safety issues concerning humans and robots.
Smart Materials Used in Soft Micro-robots
Hydrogels are hydrophilic, biocompatible and bioerodible. These characteristics are exploited in areas ranging from tissue engineering and cell scaffolding, to wound dressing and therapeutic cargo delivery. [2] By cross-linking different polymers, hydrogels can be engineered to be simultaneously highly deformable and tough, [3] which are generally considered incompatible
requirements for other materials. To make micro-robots smarter, intelligence is embedded in the materials. By combining hydrogels with stimuli-responsive components, hydrogels can be designed to adapt to their environment; regulate the transport of ions and molecules; alter their adhesion with other materials; or transform chemical and biochemical signals into optical, electrical, thermal or mechanical signals, and vice versa. [4]
The embedded intelligence in smart materials facilitates remote monitoring of patients through personal smartphones. Stimuli-responsive hydrogels coupled with a high-sensitivity inductive wireless sensor enables wireless chemical monitoring, a kind of wireless sensor network. WSNs are widely used in monitoring personal health conditions through near-field communications
connecting sensors and smartphones. [5] Different types of chemical sensors have been developed to fulfill real-time monitoring for personal daily life, such as blood oxygen, blood pressure, glucose, etc. Wireless communication bridges the gap between hospitals and patients. Information on the patients’ health condition is transferred and recorded at the health center. Therefore,
patients can be remotely diagnosed by doctors (See Figure 1).
To achieve faster diagnoses, smart materials can be equipped with more than one responsive group to attain multi-stimuli responsive properties and the detection of various physiological conditions. [6] For instance, soft micro-robots with the ability to detect autoantibodies can target specific tissues and cells and are being developed to diagnose and treat early-stage cancer. [7]
Smart hydrogels are also capable of rapid self-repair, allowing robots to recover from damage in aqueous environments. [8]
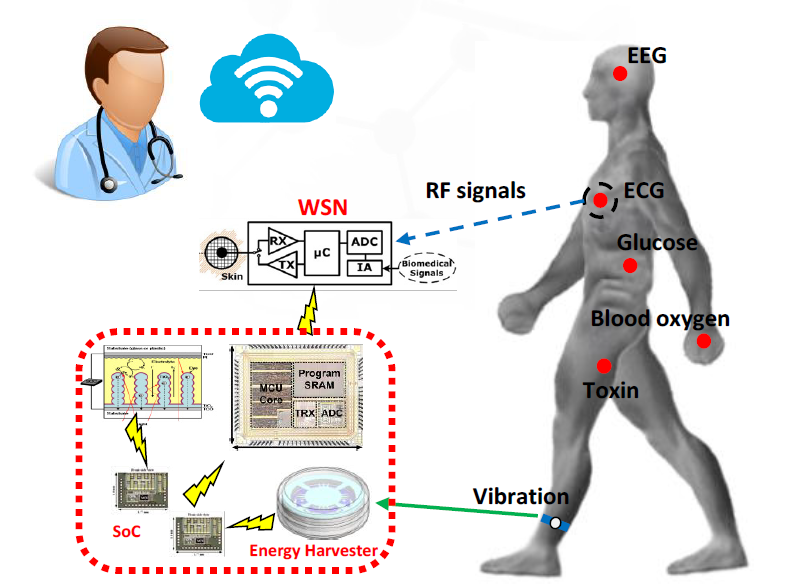
Figure 1. The concept of a wireless body sensor network.
Building Soft Micro-Robots for Therapeutic Delivery
One major advantage of forming soft micro-robots from smart materials is that such a micro-robot could automatically deliver therapy to damaged areas. This requires coordination between the embedded sensors and actuators in the devices. This level of sophistication is exhibited at this size scale by leukocytes cells, which are able to find and target pathogens. In a similar manner, smart materials can sense variations in the environment and then respond with a programmed action. For example, a micro-gripper can be programmed to open and deliver its payload only when it senses the target. [9,10] There are many emerging technologies developed for creating 3D micro/nano mobile machines employed in minimally invasive therapies, such as 3D printing [11] and self-folding. [12] Among them, origami folding based on photolithography are the most promising for generating complex shapes and high throughput production. [13,14] Developments in foldable printed circuit boards, [15] rolled-up supercapacitors for electrostatic energy storage [16] and biodegradable circuit boards [17] will equip future soft micro-robots with sophisticated
programming akin to their macro-scale counterparts.
Self-folding is achieved by coupling two materials that swell differently when hydrated. For example, a spiral tube provides both a large volume for carrying therapeutic agents and a large surface area, when uncoiled, to disperse the agent. The reversible stimuli-responsive property of hydrogels enables self-folded micro-robots to change their shapes on demand to deliver an agent. [9,18,19,20] These tiny machines are small enough to be directly injected into the human body by a syringe to perform minimally invasive therapies. The micro-robots can either be manipulated remotely by external user control or navigate passively through blood flow. Hydrogel based micro-robots, with intrinsically soft and flexible properties, are able to access mechanical constrictions,
such as narrowed and obstructed vessels. The remote control of micro-robots can be achieved by embedding magnetic nanoparticles into polymer-based actuators. The layout of the embedded particles determines the mobility of the magnetically guided micro-robots. Alternative actuating methods, such as near-infrared light, [12] electrical fields [21] and chemical sources [22], can be realized by embedding different types of micro/nanoparticles. Advanced mobility of self-folded micro-robots has been achieved by mimicking the morphology, locomotion, and morphological
adaptation of natural microorganisms [13] (See Figure 2). Microorganisms are usually composed of a large cell body attached to tiny flagella. A synergetic motion between cell body and flagella enables exceptional motility. [23,24]
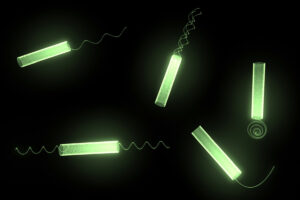
Figure 2. Bacteria inspired micro-robots are composed of a tubular body and various types of flagella.
One major disadvantage of using hydrogel- based devices as drug carriers is the leakage of drug during transportation. Soft micro-robots with multiple shape transformations can be used to reduce pharmaceutical leakage before reaching targeted areas by refolding a self-folded tube to shield the drug loaded layer from the surroundings. [25] Moreover, the foldable micro-robots enable several devices to be assembled together to achieve more efficient propulsion or multiple functionalities (See Figure 3). The release of multiple assembled tubes can be also used in extendable release. For example, some treatments require multiple doses periodically or a controlled long-term sustainable release. Nested micro-robots can achieve sequential releases of multiple drugs or varying dosages. In this way, soft-micro robots can deliver target and tailored therapies in a robust and portable package.
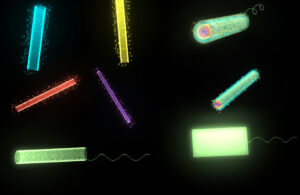
Figure 3. Matryoshka inspired micro-robots for extendable drug delivery. (a) Folded tubes loaded with different drugs. (b) Multiple folded tubes are assembled together for more efficient propulsion. (c) Chronological delivery of the therapeutic tubes by sequentially unfolding the tubes.
References
1. R. Wood and C. Walsh, “Smaller, Softer, Safer, Smarter Robots,” Science Translational Medicine, vol. 5, 2013.
2. A. S. Hoffman, “Hydrogels for biomedical applications,” Adv. Drug Deliv. Rev., vol. 54, pp. 3-12, 2002.
3. J.-Y. Sun, X. Zhao, W. R. K. Illeperuma, O. Chaudhuri, K. H. Oh, D. J. Mooney, et al., “Highly stretchable and tough hydrogels,” Nature, vol. 489, pp. 133-136, 2012.
4. M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Muller, C. Ober, M. Stamm, et al., “Emerging applications of stimuli-responsive polymer materials,” Nat. Mater., vol. 9, pp. 101-113, 2010.
5. A. Darwish and A. E. Hassanien, “Wearable and implantable wireless sensor network solutions for healthcare monitoring,” Sensors, vol. 11, pp. 5561-95, 2011.
6. P. Schattling, F. D. Jochum, and P. Theato, “Multi-stimuli responsive polymers – the allin- one talents,” Polymer Chemistry, vol. 5, pp. 25-36, 2014.
7. V. I. Butvilovskaya, S. B. Popletaeva, V. R. Chechetkin, Z. I. Zubtsova, Marya V. Tsybulskaya, L. O. Samokhina, et al., “Multiplex determination of serological signatures in the sera of colorectal cancer patients using hydrogel biochips,” Cancer Medicine, vol. 5, pp. 1361-1372, 2016.
8. A. Phadke, C. Zhang, B. Arman, C. C. Hsu, R. A. Mashelkar, A. K. Lele, et al., “Rapid self-healing hydrogels,” Proc Natl Acad Sci U S A, vol. 109, pp. 4383-8, Mar 20 2012.
9. S. Fusco, M. S. Sakar, S. Kennedy, C. Peters, R. Bottani, F. Starsich, et al., “An Integrated Microrobotic Platform for On-Demand, Targeted Therapeutic Interventions,” Adv. Mater., vol. 26, pp. 952-957, 2014.
10. J. C. Breger, C. Yoon, R. Xiao, H. R. Kwag, M. O. Wang, J. P. Fisher, et al., “Self-Folding Thermo-Magnetically Responsive Soft Microgrippers,” ACS Applied Materials & Interfaces, vol. 7, pp. 3398-3405, 2015/02/11 2015.
11. T.-Y. Huang, M. S. Sakar, A. Mao, A. J. Petruska, F. Qiu, X.-B. Chen, et al., “3D Printed Microtransporters: Compound Micromachines for Spatiotemporally Controlled Delivery of Therapeutic Agents,” Adv. Mater., vol. 27, pp. 6644-6650, 2015.
12. H.-W. Huang, M. S. Sakar, K. Riederer, N. Shamsudhin, A. Petruska, S. Pan, et al., “Magnetic microrobots with addressable shape control,” in 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 2016, pp. 1719-1724.
13. H.-W. Huang, M. S. Sakar, A. J. Petruska, S. Pane, and B. J. Nelson, “Soft micromachines with programmable motility and morphology,” Nat Commun, vol. 7, 2016.
14. R. Fernandes and D. H. Gracias, “Self-folding polymeric containers for encapsulation and delivery of drugs,” Adv. Drug. Deliv. Rev., vol. 64, pp. 1579-89, 2012.
15. A. C. Siegel, S. T. Phillips, M. D. Dickey, N. Lu, Z. Suo, and G. M. Whitesides, “Foldable Printed Circuit Boards on Paper Substrates,” Advanced Functional Materials, vol. 20, pp. 28-35, 2010.
16. R. Sharma, C. C. B. Bufon, D. Grimm, R. Sommer, A. Wollatz, J. Schadewald, et al., “Large-Area Rolled-Up Nanomembrane Capacitor Arrays for Electrostatic Energy Storage,” Advanced Energy Materials, vol. 4, pp. n/a-n/a, 2014.
17. S.-W. Hwang, H. Tao, D.-H. Kim, H. Cheng, J.-K. Song, E. Rill, et al., “A Physically Transient Form of Silicon Electronics,” Science, vol. 337, pp. 1640-1644, 2012.
18. S. IPedron, S. van Lierop, P. Horstman, R. Penterman, D. J. Broer, and E. Peeters, “Stimuli Responsive Delivery Vehicles for Cardiac Microtissue Transplantation,” Adv. Funct. Mater., vol. 21, pp. 1624-1630, 2011.
19. S. Fusco, H.-W. Huang, K. E. Peyer, C. Peters, M. Häberli, A. Ulbers, et al., “Shape-Switching Microrobots for Medical Applications: The Influence of Shape in Drug Delivery and Locomotion,” ACS Appl. Mater. Interfaces, vol. 7, pp. 6803-6811, 2015.
20. M. Jamal, S. S. Kadam, R. Xiao, F. Jivan, T. M. Onn, R. Fernandes, et al., “Bio-origami hydrogel scaffolds composed of photocrosslinked PEG bilayers,” Adv Healthc Mater, vol. 2, pp. 1142-50, Aug 2013.
21. E. Palleau, D. Morales, M. D. Dickey, and O. D. Velev, “Reversible patterning and actuation of hydrogels by electrically assisted ionoprinting,” Nat Commun, vol. 4, p. 2257, 2013.
22. V. Magdanz, G. Stoychev, L. Ionov, S. Sanchez, and O. G. Schmidt, “Stimuli-responsive microjets with reconfigurable shape,” Angew Chem Int Ed Engl, vol. 53, pp. 2673- 7, Mar 3 2014.
23. B. Liu, M. Gulino, M. Morse, J. X. Tang, T. R. Powers, and K. S. Breuer, “Helical motion of the cell body enhances Caulobacter crescentus motility,” Proc. Natl. Acad. Sci. USA, vol. 111, pp. 1252-11256, 2014.
24. Y. Kinosita, N. Uchida, D. Nakane, and T. Nishizaka, “Direct observation of rotation and steps of the archaellum in the swimming halophilic archaeon Halobacterium salinarum,” Nat Microbiol, vol. 1, p. 16148, 2016.
25. H.-W. Huang, A. J. Petruska, M. S. Sakar, M. Skoura, F. Ullrich, Q. Zhang, S. Pane, and B. J. Nelson, “Self-Folding Hydrogel Bilayer for Enhanced Drug Loading, Loading, Encapsulation, and Transport,” 38th Annual International Conference of the Engineering in Medicine and Biology Society, Orlando, FL USA (EMBC’16).


