Spinal cord injuries (SCI) occur frequently in combat. One study, completed in 2015, indicated that the incidence of combat spinal trauma during the conflicts in Iraq and Afghanistan may range from 7.4 percent to 12 percent of all warfighter injuries [1, 2]. In both military and civilian-related cases of SCI, most patients do not end up with a completely transected spinal cord. However, more than 50 percent of these patients completely lose muscle control and sensation below the injury level [3, 1]. This suggests that spared connections in incompletely transected spinal cords are functionally dormant.
As we researched strategies to turn on these spared circuits, we discovered that enhancing functions of the potassium chloride (K+/Cl-) co-transporter 2 (KCC2) protein—by either pharmacological or gene therapy approaches— is able to activate intraspinal relay pathways and lead to functional recovery in a mouse model of SCI [1]. These findings suggest a new strategy for improving recovery after SCI in humans.
Challenges
Functional deficits associated with SCI result from damaged anatomical connections and failed information exchange between the brain and the portion of the spinal cord below the lesion [4]. Therefore, a large focus of research and development in the field has been to develop strategies that promote nerve regeneration to rebuild the lost connections [5, 6].
However, it is known that in many patients with full functional deficits, not all connections are severed—raising the question of why such spared connections are non-functional. Interestingly, clinical studies have shown that when combined with rehabilitative training, electrical spinal stimulation applied to the spinal cord can result in a certain degree of voluntary functional recovery in chronically-paralyzed patients, likely by activating the dormant spared connections [7, 8]. However, once the stimulation is removed, the recovered function immediately disappeared. Thus, it is crucial to understand why the spared spinal circuitry is dysfunctional after a spinal cord injury and how it can be more persistently reactivated.
Non-biased Screening for Function-Enhancing Small Molecule Compounds
SCI triggers numerous alterations in the neuronal circuits in and out of the spinal cord. In light of the success of electrical stimulation, we reasoned that altering neuronal excitability might be a way to activate the dormant connections. Thus, we conducted a small-scale compounds screening in staggered lesioned mice with two hemisections at thoracic level T10 and, contralaterally, at T7. This model spares axons crossing the midline between T7 and T10, but still results in hindlimb paralysis [9, 10]. Among all compounds tested, we found that treatment of CLP290, a KCC2 agonist [11], for 6–8 weeks best restored weight-bearing stepping ability in paralyzed mice (see Figure 1). Such treatment failed to improve functions in mice with total transection at T8, suggesting that it likely targets the dormant intraspinal relay pathways.
KCC2-based Gene Therapy Promotes Functional Recovery
Previous studies conducted by other research groups have observed KCC2 down-regulation in injured spinal cords [12, 13], but KCC2’s relevance to functional deficits and recovery has not been formally tested. To fill this gap, we used viral vector-based strategies to over-express KCC2 in different groups of neurons and assessed the functional outcomes. We found that non-selective expression of KCC2 in all neurons of the brain and spinal cord mimics the effects of CLP290.
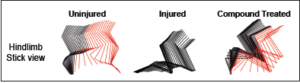
Figure 1. Color-coded stick view decomposition of mouse hindlimb movements during walking (uninjured), dragging (injured) and stepping (compound treated injured group) [4].
Strikingly, selective expression of KCC2 in inhibitory interneurons between and around the staggered spinal lesions was sufficient to achieve improved functional recovery in injured mice with double hemisection at T7 and T10. These data suggest that the downregulation of KCC2 in inhibitory interneurons, which are part of the spared intraspinal relays, renders the circuit dysfunctional and prevents functional recovery in incomplete SCI. Consistent with these results, our mechanistic studies further showed that KCC2-related treatments transformed the injured spinal circuit from a mal-functional to a functional state, which in turn facilitated the transmission of brain-derived signals to the lumbar spinal cord below the lesion.
Conclusions
Our results indicate that injury-induced KCC2 down-regulation is a critical mechanism that contributes to the dysfunction of spared neuronal connections after SCI. Importantly, restoring KCC2 function could reinstate the ability of spared neuronal connections to participate in the spinal relays involved in hindlimb function.
Because KCC2 agonists could be administered systematically without detectable side effects [4, 11], this approach may have direct implications in clinical treatments of SCI. Further development of this approach may accelerate the rate at which injured warfighters recover from the debilitating effects of SCI.
Towards this goal, further studies should assess the functional outcomes of such manipulations in other injury models and seek to optimize a therapeutic regimen for possible clinical studies. As electrical stimulation may modulate neuronal excitability, it would be informative to assess (a) whether shared or distinct mechanisms underlie the action of electrical stimulation and KCC2 manipulations, and (b) whether these methods could be used in combination to further enhance the extents and/or duration of functional recovery after SCI. Finally, major advances have been made elsewhere in developing strategies to promote axon regeneration, which could be combined with KCC2-mediated modulations to maximize functional restoration after SCI.
References
1. Bernstock, J. D., Caples, C. M., Wagner, S. C., Kang, D. G., & Lehman, R. A. Jr. (2015). Characteristics of combat-related spine injuries: A review of recent literature. Military Medicine, 180(5), 503–512. doi:10.7205/MILMED-D-14-00215
2. Schoenfeld, A. J., Newcomb, R. L., Pallis, M. P., Cleveland, A. W. 3rd, Serrano, J. A., Bader, J. O., . . . Belmont, P. J. Jr. (2013). Characterization of spinal injuries sustained by American service members killed in Iraq and Afghanistan: A study of 2,089 instances of spine trauma. Journal of Trauma and Acute Care Surgery, 74(4), 1112–1118.doi:10.1097/TA.0b013e31828273be
3. Kakulas, B. A. (1999). A review of the neuropathology of human spinal cord injury with emphasis on special features. Journal of Spinal Cord Medicine, 22, 119–124. doi:10.1080/10790268.1999.11719557
4. Chen, B., Li, Y., Yu, B., Zhang, Z., Brommer, B., Williams, P. R., . . . He, Z. (2018). Reactivation of dormant relay pathways in injured spinal cord by KCC2 manipulations. Cell, 174(3), 521–535. doi:10.1016/j.cell.2018.06.005
5. Sofroniew, M. V. (2018, May). Dissecting spinal cord regeneration. Nature, 557(7705), 343–350. doi:10.1038/s41586-018-0068-4
6. He, Z., & Jin, Y. (2016, May 4). Intrinsic control of axon regeneration. Neuron, 90(3), 437–451. doi:10.1016/j.neuron.2016.04.022
7. Angeli, C. A., Edgerton, V. R., Gerasimenko, Y. P., & Harkema, S. J. (2014, May). Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain, 137(Pt 5), 1394–1409.doi:10.1093/brain/awu038
8. Harkema, S., Gerasimenko, Y., Hodes, J., Burdick, J., Angeli, C., Chen, Y., . . . Edgerton, V. R. (2011, June 4). Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet, 377(9781), 1938–1947. doi:10.1016/S0140-6736(11)60547-3
9. Courtine, G., Song, B., Roy, R. R., Zhong, H., Herrmann, J. E., Ao, Y., . . . Sofroniew, M. V. (2008, January). Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nature Medicine, 14(1), 69–74.doi:10.1038/nm1682
10. an den Brand, R., Heutschi, J., Barraud, Q., DiGiovanna, J., Bartholdi, K., Huerlimann, M., . . . Courtine, G. (2012, June 1). Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science, 336(6085), 1182–1185. doi:10.1126/science. 1217416
11. Gagnon, M., Bergeron, M. J., Lavertu, G., Castonguay, A., Tripathy, S., Bonin, R. P., . . . De Koninck, Y. (2013, November ). Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nature Medicine, 19(11), 1524–1528. doi:10.1038/nm.3356
12. Boulenguez, P., Liabeuf, S., Bos, R., Bras, H., Jean-Xavier, C., Brocard, C., . . .Vinay, L. (2010, March). Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nature Medicine, 16(3), 302–307. doi:10.1038/nm.2107
13. Cote, M. P., Gandhi, S., Zambrotta, M., & Houle, J. D. (2014, July 2). Exercise modulates chloride homeostasis after spinal cord injury. Journal of Neuroscience, 34(27), 8976–8987. doi:10.1523/JNEUROSCI.0678-14.2014


