Chemical analysis of human breath, bodily secretions, and odor signatures is an emerging focus of the biometrics and human systems research communities. As the Air Force Office of Scientific Research has noted, sweat and breath in particular are leading candidates for the development of novel biomarkers useful in multiple mission sets, in both cooperative and non-cooperative settings [1].
Our laboratories are currently investigating the change in volatile organic compounds (VOCs) from the oral cavity when participants are in different states of psychological arousal and emotional states, with the goal of identifying a set of VOCs as a biometric reliably associated with stress in individuals. To date, our findings demonstrate that states of psychological arousal reliably change the volatile organic compound (VOC) or biometric chemical signatures that are produced in both breath and bodily secretions. The ability to rapidly and non-invasively detect whether an individual is psychologically stressed could provide numerous analytical and operational benefits to the Department of Defense (DoD) and federal agencies charged with homeland defense missions.
Sensor-driven instrumentation for the detection and identification of stressed individuals from VOC signatures could advance DoD capabilities in several areas. One, VOC sensors calibrated to detect stress compounds could facilitate the detection and identification of stressed (and potentially dangerous) individuals at entry points to critical facilities, complementing or improving current methods of (a) flagging potential false utterances via thermal infrared imaging [2, 3] or (b) observing and interpreting behavioral indicators of stress and duplicity. Two, odors from human breath could be used to complement other biometric characteristics in continuous identity authentication systems [4]. Three, physiological measures of psychological stress levels could advance the medical monitoring of warfighters. Current means rely on self-reporting of stress severity, which can vary across contexts and time (and can return undependable data in a culture where individuals are trained to compete for optimal performance) [5, 6], and semi-invasive physiological testing methods [7].
Background
The Stress Response and Stress Odors
Animal models have demonstrated that a state of stress produces a distinctive odor [8, 9] that can be detected by conspecifics and affect the behavior and physiology of recipients [10–14]. Research conducted by our laboratories and others has demonstrated that this is also true for humans, with evidence for the ability to discriminate stress from non-stress body odor and for stress sweat to have an effect on task performance and brain activity.
Animal models have demonstrated that a state of stress produces a distinctive odor [8, 9] that can be detected by conspecifics and affect the behavior and physiology of recipients [10–14]. Research conducted by our laboratories and others has demonstrated that this is also true for humans, with evidence for the ability to discriminate stress from non-stress body odor and for Stress-related odors are thought to arise from an increased production of body odorants mediated by increases in stress-related hormones. However, the chemical identity of these odorants has not been determined. In humans, the autonomic nervous system (ANS) is responsible for producing a stress response triggered by the hypothalamus, sending hormonal signals to the pituitary and adrenal glands. Characteristic physiological changes that ensue include:
- increased heart rate
- increased blood supply
- decreased salivary flow
- increased secretions from eccrine and apocrine sweat glands
These changes are thought to occur as a result of the increased production of stress-related hormones, such as epinephrine (adrenaline) and cortisol, and their effect on odor-producing body structures, such as eccrine and apocrine glands found in the underarm [15] and salivary glands in the mouth.
To experimentally induce psychological stress, we employed a standard laboratory stress task, known as the Trier Social Stress Test (TSST) [16, 17]. In this test, subjects are required to engage in a public speaking task and a mental subtraction task while being observed and evaluated. The test has been widely validated and shown to produce reliable increases in heart rate, skin conductance response, and stress hormones—allowing us to collect samples that can be reliably paired with physiological indices of stress.
Underarm Odor
Studies focusing on human underarm sweat have shown that sweat sampled from a cohort of donors while experiencing a form of psychological stress can change the task performance [18–25] and brain activity [26–29] of a separate set of participants who are exposed to this “stress sweat” as an odor stimulus, as compared against a control stimulus (neutral, baseline, or mechanical, exercise-induced sweat). Exposure to stress sweat has been demonstrated to affect a wide range of behaviors, including:
- changing perceived competence, confidence, and trustworthiness of women [18]
- potentiating the startle reflex [19, 23]
- modulating fear recognition in faces [20]
- inducing empathy [21] and anxiety [24] in the participants who are exposed to the sweat
The neurological underpinnings of the effect of smelling stress sweat have also been explored in depth [27], with studies showing its effect on the neural processing of neutral faces [29] as well as activation of the amygdala [26].
Our own laboratories have demonstrated that human evaluators can reliably discriminate stress sweat from non-stress sweat [33]. Eighteen donor participants (13 male and five female) engaged in a washout week—using only unfragranced soaps and prohibiting colognes or fragranced products. This continued during the collection of underarm samples before and after both a neutral and stress manipulation task.
These samples were pooled together and presented in a counterbalanced manner to a separate cohort of evaluator participants, screened for olfactory sensitivity, in a three-alternative forced-choice (i.e., triangle test) method in which two samples were the same and one was different.
We evaluated participants’ ability to detect the “different” sample when comparing various combinations of the following sweat samples:
- pre-neutral
- post-neutral
- pre-stress
- post-stress
A Chi-Square analysis revealed that although no differences were seen when comparing the pre- and post-neutral session samples, χ2 (2, N=18) = 2.03, p=0.36, statistically significant differences were seen both when looking at post-neutral and post-stress samples, χ2 (2, N=18) = 52.08, p<0.001. This is compelling evidence that humans can detect a difference when comparing stress-sweat samples with samples collected under non-stressed conditions. Unfortunately, few of these studies include a chemical analysis of the sweat samples, leaving open the question of which VOCs are changing in the sweat from one state of arousal to another. The unique combination of expertise in our studies allows us to investigate both the psychological and physiological markers of stress and the analytical evidence of changes in the VOCs emitted.
Table 1. Compounds analyzed in breath and their proposed origins. * Indicates microbiome-related odors removed from analysis
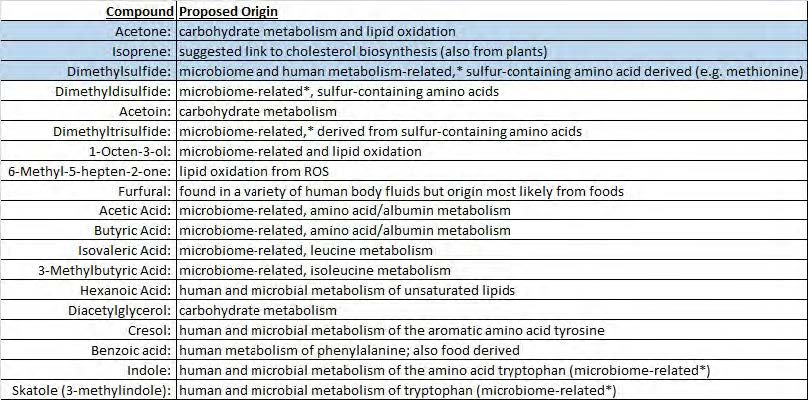
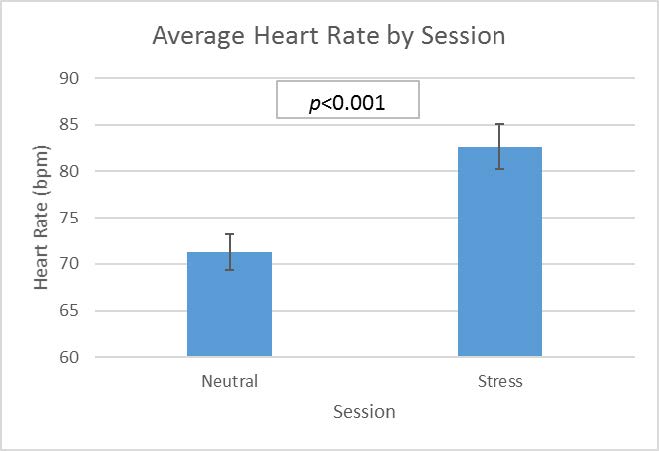
Figure 1. Heart rate change in subjects during neutral and stress conditions
Breath and Saliva
With support from the Air Force Office of Scientific Research, our laboratories have worked towards finding biomarkers for psychological stress that are reliable and more accessible than underarm sweat samples. We focused on the oral cavity, which provides two abundant and easy-to-collect samples to be used for chemical analysis: breath and saliva.
Previous research using human subjects has demonstrated that stress-related hormonal changes from either physiological [34] or psychological stress [35] create alterations in the oral cavity—perhaps tied to alterations in salivary flow and composition—which consequently upregulate production of specific VOCs, such as volatile sulfur compounds, and possibly oxygenated compounds related to oxidative stress. Based on these previous results, we hypothesized that analysis of expired breath and saliva would yield VOCs characteristic of stress states.
To test our hypothesis, we collected breath and saliva samples from human subjects before and after inducing psychological stress with the TSST.
Method
Participants began the study by taking part in three to five days of an oral “washout” period during which they used a laboratory-provided, non-flavored toothpaste; refrained from using mouthwash; and maintained a bland diet devoid of foods that contribute to oral odors (e.g., garlic, onions, curries). Participants then came in for two sessions of testing, on separate days, while maintaining their oral washout regimen.
The sessions started with a collection of saliva and breath samples before the recording of baseline physiological measures (e.g., heart rate). Participants were then either presented with a 15-minute DVD of cinematic landscape videos (neutral session), or the TSST (stress session). Physiological measures were collected throughout the manipulations (both neutral and stress sessions) with breath and saliva samples collected after each manipulation.
From this dataset, we did a manipulation check with an analysis on heart rate change from baseline—a solid indicator of stress as a byproduct of adrenaline release—and excluded any participant whose heart rate did not increase from baseline during the stress session. This ensured that samples collected from these individuals were not included in the chemical analysis, thereby guaranteeing that all samples used for analysis were from quantifiably stressed individuals. This left us with a pool of 46 participants (27 female and 19 male), with an average age of 26.9 years.
Participants provided breath samples by blowing into a 1L ALTEF polypropylene bag (Restek, Bellefonte, PA) until filled, and saliva samples by chewing unflavored gum base (Wrigley Company, Chicago, IL) and expectorating saliva into a 20 ml tube at one-minute intervals for three minutes. VOCs for each breath sample were collected using solid-phase micro-extraction (SPME), then separated and analyzed by gas chromatography–mass spectrometry (GC/MS).
VOCs from each saliva sample were collected after incubating a 1 ml aliquot of each sample for 30 minutes, via magnetic stirring, at 37 degrees Celsius in a 4 ml glass vial fitted with a silicone/tetrafluoroethylene septum-containing screw cap to enhance the headspace VOC content. A SPME fiber was then inserted into the vial and VOCs collected for 30 minutes, followed by injection into the GC/MS for separation and identification. A variety of compounds were identified (see Table 1) and their relative amounts were manually calculated prior to statistical analyses.
Results
Stress Manipulation
As a check for the stress manipulation, we looked at heart rate as an indication of stress during both the neutral and stress manipulations. As Figure 1 indicates, participants experienced a significantly higher heart rate during the stress session than during the neutral session.
VOC Analysis
Several of the compounds listed in Table 1 were clearly seen in selected individuals but not in others (e.g., skatole, dimethyl di-, and dimethy trisulfide), and were not included in our analyses; we made the assumption that these compounds were derived from the tongue microbiome and associated with putative breath odor problems.
For the remaining compounds, a repeated-analysis of variance (ANOVA) of the breath samples demonstrated that the concentration of these compounds in poststress samples was significantly greater than pre-neutral samples (p < 0.05). Post-hoc analysis (Bonferroni corrected) showed that this difference is mainly driven by acetone (p < 0.001) and isoprene (p < 0.05).
When we further examined each of the individual VOCs using ANOVA, three of the volatiles showed a statistically significant difference from pre-neutral to post-stress at the 0.05 alpha level: acetone and isoprene (as predicted and demonstrated by the above post-hoc analysis), as well as dimethyl sulfide (see Table 2). While this suggests dimethyl sulfide as a putative stress biomarker, more testing is required for a definitive confirmation. In contrast, salivary levels of these compounds were not significantly altered, suggesting a nonsalivary origin.
VOCs and Physiological Measures
We next examined the relationships between the VOCs that were significantly altered by stress and subjects’ physiological stress measures. As shown in Figure 2, acetone was positively correlated with heart rate, p < 0.05, but not with post-stress cortisol measures, whereas both isoprene (p < 0.05) and diemthylsulfide (p < 0.05) showed significant positive correlations with post-stress cortisol measures as seen in Figures 3 and 4 (respectively), but not with heart rate.
Conclusions
Physiological changes documented in our subjects demonstrated that the stress induction was effective. Analytically, three volatile organic compounds found in breath were found to be significantly elevated by stress: acetone, isoprene, and dimethylsulfide. In contrast, the concentration of these compounds was not significantly elevated in saliva. This suggests that these compounds are not formed in saliva, but are produced by changes in metabolic processes in either the kidneys or liver. Dimethylsulfide may also originate from the oral microbiome, but it is unlikely that a single episode of psychological stress would affect this.
The data show that the breath-borne biomarkers which increase significantly with stress may not all be dependent on the same aspects of the stress response. Increased heart rate was correlated with acetone and is part of the “fast, short term stress response” (also known as the fight-or-flight response) associated with a release of adrenalin/epinephrine. Release of epinephrine also causes the liver to release glucose, which in turn may account for the significant increase in breath acetone. Longer-term stress response is associated with cortisol release, which our data show are correlated with dimethylsulfide and isoprene.
Although our preliminary data show promise, further research on the reliability of these compounds to predict and indicate individual states of psychological stress are needed to realize the ability to identify a psychologically stressed and potentially dangerous suspect or target or to monitor warfighters’ stress levels. Once a set of compounds are established as reliable indicators of stress, they may be transitioned into nano-enabled sensor systems fitted to the face masks of pilots or headset-microphones of deployed ground personnel [36–37]. These sensor systems, calibrated to detect the presence and concentration of stress-related VOCs in exhaled breath, could provide real-time, non-invasive individual monitoring of physiological status to allow for continuous evaluation of warfighter stress. In addition, sensors systems calibrated to detect stress may be fitted to hand-held devices that could be employed by personnel at critical points of entry or secured buildings to detect stressed or deceptive individuals. This technology could deliver a significant improvement to the “suspicious individual” detection methods currently used that rely primarily on a set of behavioral indicators to identify persons who may pose a security risk.
Table 2. Results of ANOVA for three breath VOCs
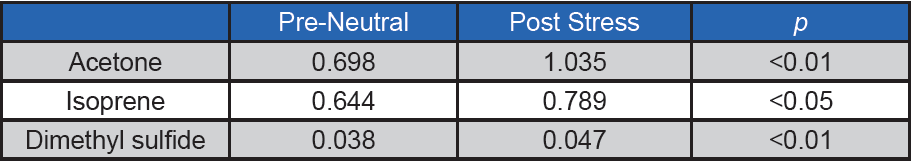
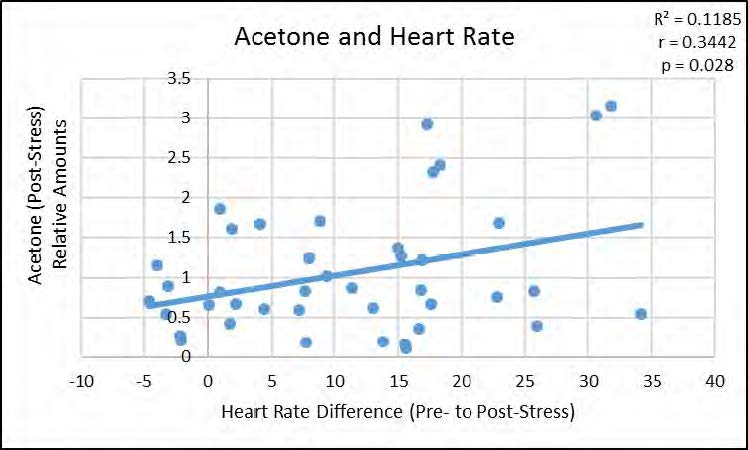
Figure 2. Significant positive correlation between relative amounts of Acetone in breath, poststress, and heart rate difference from baseline to post-stress
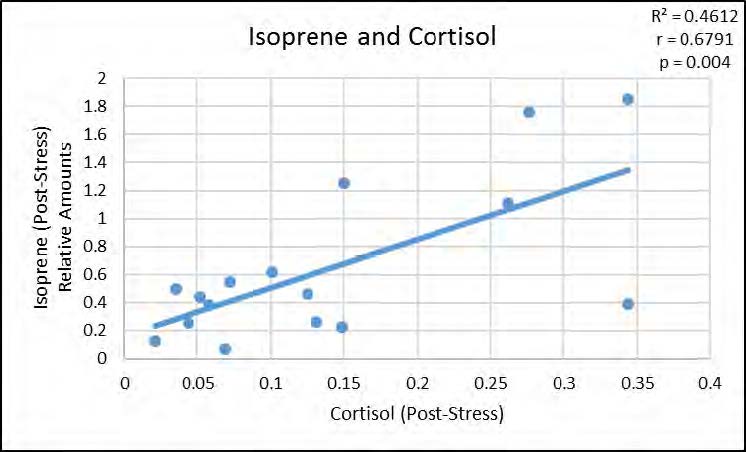
Figure 3. Significant positive correlation between relative amounts of Isoprene in breath, poststress, and post-stress cortisol levels
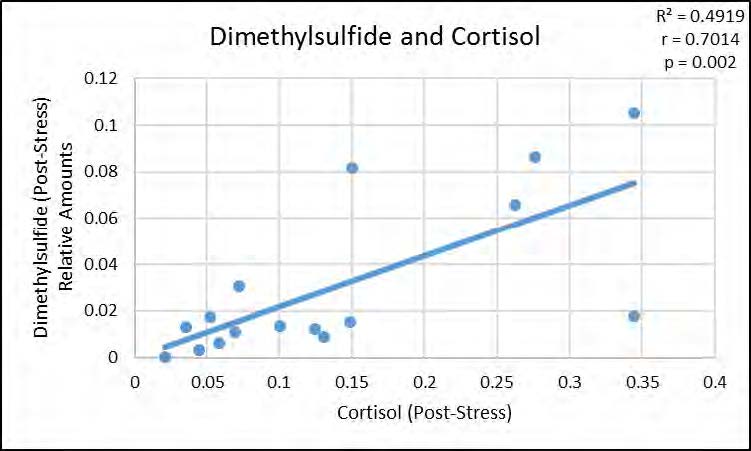
Figure 4. Significant positive correlation between relative amounts of Dimethylsulfide in breath, post-stress, and post-stress cortisol levels
References
1. Bradshaw, P. O. (2014, March). Hu- man performance and biosystems. Air Force Office of Scientific Research, Air Force Research Laboratory. Retrieved from https://apps.dtic.mil/dtic/tr/fulltext/ u2/1013090.pdf
2. Warmelink, L., Vrij, A., Mann, S., Leal, S., Forrester, D., & Fisher, R. P. (2011, Feb- ruary). Thermal imaging as a lie detection tool at airports. Law and Human Behav- ior, 35(1), 40–48. doi:10.1007/s10979- 010-9251-3
3. Coffey, V. C. (2015, October 1). Hyper- spectral imaging for safety and security. Optics & Photonics News. Retrieved from https://www.osa-opn.org/opn/media/Im- ages/PDF/2015/1015/26-33-Hyperspec- tral-new.pdf?ext=.pdf
4. Wu, G., Wang, J., Zhang, Y., & Jiang, S. (2018, January 10). A continuous identity authentication scheme based on physi- ological and behavioral characteristics. Sensors, 18(179), 1–20. doi:10.3390/ s18010179
5. Morgan, E.S., Umberson, K., & Hertzog, C. (2014, March). Construct validation of self-reported stress scales. Psychological Assessment 26(1), 90–99. doi:10.1037/ a0034714
6. Walentynowicz, M., Schneider, S., & Stone, A. A. (2018, August 9). The effects of time frames on self-report. PLoS One, 13(8). doi:10.1371/journal.pone.0201655
7. Sood, P., Priyadarshini, S., & Aich, P. (2013, May 15). Estimation of psycho- logical stress in humans: A combination of theory and practice. PloS One, 8(5). doi:10.1371/journal.pone.0063044
8. Mackay-Sim, A., & Laing, D. G. (1981). The sources of odors from stressed rats. Physiology & Behavior, 27(3), 511–513. doi:10.1016/0031-9384(81)90340-1
9. Rottman, S. J., & Snowdown, C. T. (1972). Demonstration and analysis of an alarm pheromone in mice. Journal of Comparative and Physiological Psycholo- gy, 81(3), 483. doi:10.1037/h0033703
10. Fanselow, M. S. (1985). Odors released by stressed rats produce opioid analgesia in unstressed rats. Behavioral Neurosci- ence, 99(3), 589–600. doi:10.1037/0735- 7044.99.3.589
11. Cocke, R., Moynihan, J. A., Cohen, N., Grota, L. J., & Ader, R. (1993). Exposure to conspecific alarm chemosignals al- ters immune responses in BALB/c mice. Brain, Behavior, and Immunity, 7(1), 36– 46. doi:10.1006/brbi.1993.1004
12. Moynihan, J. A., Karp, J. D., Cohen, N., & Ader, R. (2000). Immune deviation following stress odor exposure: role of endogenous opioids. Journal of Neuroim- munology, 102(2), 145–153. doi:10.1016/ S0165-5728(99)00173-3
13. Kiyokawa, Y., Kikusui, T., Takeuchi, Y., & Mori, Y. (2004). Alarm pheromones with different functions are released from dif- ferent regions of the body surface of male rats. Chemical Senses, 29(1), 35–40. doi:10.1093/chemse/bjh004
14. Kiyokawa, Y., Kikusui, T., Takeuchi, Y., & Mori, Y. (2005). Alarm pheromone that aggravates stress-induced hyperthermia is soluble in water. Chemical Senses, 30(6), 513–519. doi:10.1093/chemse/ bji044
15. Montagna, W., Parakal, P. F. (1974). Apocrine Glands. In W. Montagna (Ed.), The Structure and Function of Skin (pp. 332–365). New York and London: Aca- demic Press.
16. Kirschbaum, C., Pirke, K. M., & Hellham- mer, D. H. (1993). The ‘Trier Social Stress Test–A tool for investigating psychobio- logical stress responses in a laboratory setting. Neuropsychobiology, 28(1-2), 76–81. doi:10.1159/000119004
17. Smyth, J., Ockenfels, M. C., Porter, L., Kirschbaum, C., Hellhammer, D. H., & Stone, A. A. (1998, May). Stressors and mood measured on a momentary ba- sis are associated with salivary cortisol secretion. Psychoneuroendocrinology, 23(4), 353–370. doi:10.1016/S0306- 4530(98)00008-0
18. Dalton, P., Mauté, C., Jaén, C., & Wil- son, T. (2013). Chemosignals of stress influence social judgments. PloS One, 8(10), e77144. doi:10.1371/journal. pone.0077144
19. Prehn, A., Ohrt, A., Sojka, B., Ferstl, R., & Pause, B. M. (2006). Chemo- sensory anxiety signals augment the startle reflex in humans. Neuroscience Letters, 394(2), 127–130. doi:10.1016/j. neulet.2005.10.012
20. Zhou, W., & Chen, D. (2008, October). Chemosignal of fear modulates fear rec- ognition in ambiguous facial expressions. In List of Abstracts from the 15th Inter- national Symposium on Olfaction and Taste. Chemical Senses, 33(8), S175. doi:10.1093/chemse/bjn065
21. Prehn-Kristensen, A., Wiesner, C., Berg- mann, T. O., Wolff, S., Jansen, O., Me- hdorn, H. M., … & Pause, B. M. (2009). Induction of empathy by the smell of anx- iety. PloS One, 4(6), e5987. doi:10.1371/ journal.pone.0005987
22. Chen, D., Katdare, A., & Lucas, N. (2006). Chemosignals of fear enhance cognitive performance in humans. Chemical Sens- es, 31(5), 415–423. doi:10.1093/chemse/ bjj046
23. Pause, B. M., Adolph, D., Prehn-Kris- tensen, A., & Ferstl, R. (2009). Startle response potentiation to chemosensory anxiety signals in socially anxious indi- viduals. International Journal of Psycho- physiology, 74(2), 88–92. doi:10.1016/j. ijpsycho.2009.07.008
24. Albrecht, J., Demmel, M., Schöpf, V., Kl- eemann, A. M., Kopietz, R., May, J., … & Wiesmann, M. (2010). Smelling che- mosensory signals of males in anxious versus nonanxious condition increases state anxiety of female subjects. Chem- ical Senses, 36(1), 19–27. doi:10.1093/ chemse/bjq087
25. de Groot, J. H., Smeets, M. A., Kalde- waij, A., Duijndam, M. J., & Se- min, G. R. (2012). Chemosignals communicate human emotions. Psy- chological Science, 23(11), 1417–1424. doi:10.1177/0956797612445317
26. Mujica-Parodi, L. R., Strey, H. H., Fred- erick, B., Savoy, R., Cox, D., Botanov, Y., … & Weber, J. (2009). Chemosenso- ry cues to conspecific emotional stress activate amygdala in humans. PLoS One, 4(7), e6415. doi:10.1371/journal. pone.0006415
27. Lundström, J. N., Boyle, J. A., Zatorre, R. J., & Jones-Gotman, M. (2007). Func- tional neuronal processing of body odors differs from that of similar common odors. Cerebral Cortex, 18(6), 1466–1474. doi:10.1093/cercor/bhm178
28. Sobel, N., Prabhakaran, V., Hartley, C. A., Desmond, J. E., Glover, G. H., Sullivan, E. V., & Gabrieli, J. D. (1999). Blind smell: Brain activation induced by an undetect- ed air-borne chemical. Brain, 122(2), 209–217.
29. Rubin, D., Botanov, Y., Hajcak, G., & Mujica-Parodi, L. R. (2012, February). Second-hand stress: Inhalation of stress sweat enhances neural response to neutral faces. Social Cognitive and Af- fective Neuroscience, 7(2), 208–212. doi:10.1093/scan/nsq097
30. Chen, D., & Haviland-Jones, J. (2000). Human olfactory communica- tion of emotion. Perceptual and Motor Skills, 91(3), 771–781. doi:10.2466/ pms.2000.91.3.771
31. Ackerl, K., Atzmueller, M., & Grammer, K. (2002). The scent of fear. Neuroendocri- nology Letters, 23(2), 79–84.
32. McClintock, M. K., Bullivant, S., Jacob, S., Spencer, N., Zelano, B., & Ober, C. (2005, January). Human body scents: Conscious perceptions and biological effects. Chemical Senses, 30(suppl_1), i135–i137. doi:10.1093/chemse/bjh151
33. Dalton, P., Maute, C., Preti, G. (2014, April) Psychosocial Stress in Humans Produces an Axillary Odor Signature. Poster presented at the 36th Annual Meeting of the Association for Chemore- ception Science, Bonita Springs, FL.
34. Queiroz, C. S., Hayacibara, M. F., Tab- choury, C. P. M., Marcondes, F. K., & Cury, J. A. (2002, September 18). Re- lationship between stressful situations, salivary flow rate and oral volatile sulfur‐ containing compounds. European Jour- nal of Oral Sciences, 110(5), 337–340. doi:10.1034/j.1600-0722.2002.21320.x
35. Tonzetich, J., Preti, G., & Huggins, G. R. (1978). Changes in concentration of volatile sulphur compounds of mouth air during the menstrual cycle. Journal of In- ternational Medical Research, 6(3), 245– 254. doi:10.1177/030006057800600313
36. Johnson, A.T.C., Kahmis, S.M., Preti, G., Kwak, J. and Gelperin, A. (2010). DNA-coated nanosensors for breath anal- ysis. IEEE Sensor Journal 10(1), 159– 166. doi:10.1109/JSEN.2009.2035670
37. Kybert.J., Egan, L., Waldman, R.Z., Zeng, X-N, Krein, M, Preti, G., . . . & Johnson, A.T.C. (2014, May 19). Anal- ysis of sweat simulant mixtures using multiplexed arrays of DNA-carbon nano- tube vapor sensors. Journal of Foren- sic Science and Criminology, 1(1), 1–6. doi:10.15744/2348-9804.1.S102


