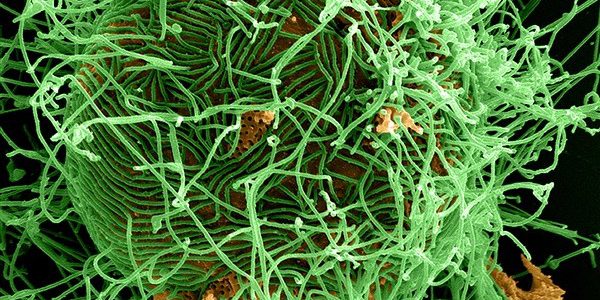
Ebola Hemmorhagic Fever (Ebola HF) or Ebola Virus Disease (EVD) is a severe and often fatal viral hemorrhagic fever disease of humans and other primates. Case fatality rates vary from 50 to 90 percent in humans.[1] The name “Ebola” recognizes the 1976 discovery of the Ebola virus near the Ebola River (two simultaneous outbreaks in Nzara, Sudan and Yambuku, Democratic Republic of Congo). Periodic outbreaks of the disease suggesting crossover into a new human host from a natural reservoir (which may include indigenous bat populations) have been observed since that time, leading up to the most severe outbreak on record, which currently involves populations in Guinea, Liberia and Sierra Leone. [2] Ebola HF illustrates an important insight in medical virology: most pathogenic viral infections are often associated with recent inter-species crossover from an evolved and adapted host-viral interaction (Ebola virus – Bat) to infection of a new host (Ebola virus – Human).
One of the most transmissible of the viral hemorrhagic fevers, Ebola HF is caused by infection with a virus of the family Filoviridae, genus Ebolavirus. Five subspecies of Ebola virus have been identified, four of which cause disease in humans: Ebola virus (Zaire ebolavirus); Sudan virus (Sudan ebolavirus); Taï Forest virus (Taï Forest ebolavirus, formerly Côte d’Ivoire ebolavirus); and Bundibugyo virus (Bundibugyo ebolavirus). The fifth, Reston virus (Reston ebolavirus), has caused disease in nonhuman primates but not in humans. Marburg virus is a closely related filovirus that shares many viral and pathophysiologic characteristics with Ebola virus. The natural reservoir of Ebola virus remains unproven, but current evidence suggests zoonotic host(s) native to the rain forests of Africa and the Western Pacific.
Ebola Hemmorhagic Fever Key Facts [3]
- Ebola virus disease (EVD), formerly known as Ebola hemor-rhagic fever, is a severe, often fatal illness in humans.
- EVD outbreaks have a case fatality rate of up to 90 percent.
- EVD outbreaks occur primarily in remote villages in Central and West Africa, near tropical rainforests.
- The virus is transmitted to people from wild animals and spreads in the human population through human-to-human transmission.
- Fruit bats of the Pteropodidae family are considered to be the natural hosts of the Ebola virus.
- Severely ill patients require intensive supportive care. No licensed specific treatment or vaccine is available for use in people or animals.

Straw-colored fruit bats, Eidolon helvum (shown), and other bat species may have carried Ebola virus from Central Africa to West Africa, where the virus is now causing the largest-ever epidemic of the disease. (Courtesy of Fritz Geller-Grimm/Released)
African fruit bats are considered possible natural hosts for Ebola virus (genera Hypsignathus monstrosus, Epomops franqueti and Myonycteris torquata), and the geographic distribution of Ebola viruses may overlap with the range of the fruit bats. Human-to-human transmission of the Ebola virus is primarily associated with direct or indirect contact with blood and body fluids. Transmission to health-care workers has been observed when appropriate infection control measures have not been followed.
Clinical features of Ebola HF begin with a prodrome (early symptom) period of 2 to 21 days, which then steadily progresses to yield a characteristic cluster of physical symptoms and manifestations coupled with a flat affect and sunken eyes often referred to as “mask like” or “ghost like.” [4] During the prodrome period, symptoms are non-specific and can be easily misdiagnosed or overlooked by medical professionals, public health workers and safety specialists such as those responsible for customs and border protection and for screening international air travelers.
Causes of Naturally Occurring Viral Hemorrhagic Fever
Ebola Virus: Natural reservoir and reservoir – human transmission unknown
Marburg Virus: Natural reservoir and reservoir – human trans-mission unknown
Lassa Virus: Rodents are primary natural reservoir
New World Viral Hemorrhagic Fevers: Multiple viral agents, rodent reservoirs, transmission to humans typically via contact or intake of rodent fecal material (including dust)
Rift Valley Fever: Mosquito borne disease of mammals, including humans
Yellow Fever: Mosquito vector-borne disease of humans
Dengue Hemorrhagic Fever: Mosquito vector-borne disease of humans
After the clinical disease declares itself, initial prodromic symptoms typically progress over four to five days to a general toxic presentation with more pronounced fever, severe myalgia (including pain on palpation), arthralgia (joint pain) and prominent gastrointestinal symptoms, which may include vomiting coupled with colicy abdominal pain and profound anorexia. A papular or maculo-papular rash of the upper arms, flexor surfaces of the forearms and upper leg often develops and may progress to desquamation (shedding outer skin layers) in the same distribution as well as palms and soles of the extremities.
Hemorrhagic bleeding diatheses (predispositions) include gastrointestinal (watery diarrhea with fresh blood, melenotic stools, vomiting of fresh blood), naso-oropharyngeal (mouth, gums, nasopharynx), ocular (subconjunctival) and genital (vaginal) presentations. Common early central nervous symptoms include stiff neck, which is common to meningitis but with clear cerebrospinal fluid, and as the disease progresses patients often exhibit bizarre behavior, including a tendency to leave the place of care and wander in a confused state. Severe cases progress to death from 2 to 21 days after clinical presentation, with a peak at day nine.
Initial diagnosis of Ebola HF presenting early in the clinical progression in the absence of a documented outbreak can be challenging. The differential diagnosis includes many disease also found in edge environments associated with the sporadic pattern of Ebola HF. Disease presenting with similar symptom clusters include the following: [5]
Bacterial and Rickettsial Infections:
Gram-negative bacterial septicemia
Staphylococcal or streptococcal toxic shock syndrome
Meningococcemia
Secondary syphilis
Septicemic plague
Typhoid fever
Rocky Mountain spotted fever
Ehrlichiosis
Leptospirosis
Viral and Parasitic Infections:
Malaria
African trypanosomiasis
Hemorrhagic smallpox
Measles
Hemorrhagic varicella
Rubella
Viral hepatitis
Other Conditions:
Thrombotic or Idiopathic thrombocytopenic purpura
Acute leukemia
Hemolytic uremic syndrome

Surrounded by Ebola patients, a health worker (center) gives thumb ups to visitors near the hot zone. The hot zone is defined by the double barrier orange fence in the event a sick person falls, they cannot contaminate the clean zone. (U.S. Army Africa photo by Cmdr. Peter Niles/ Released)
Ebola HF Prodrome Symptoms
- Progressive, febrile “flu-like” illness
- Can be mistaken for malaria or non-specific viral syndrome
- Chief complaints include:
- Mild fever between 100 and 102 F
- Severe headache
- Generalized myalgia, arthralgia
- Dry, sore throat
- Pleuritic chest pain with dry cough
Diagnostic Signs of Ebola Hemmorhagic Fever[5]
- Acute onset fever (<3 weeks duration)
- Severe prostrating or life-threatening illness
- Bleeding manifestations (at least two of the following: hemor-rhagic or purpuric popular rash, epistaxis, hematemesis, hem-optysis, blood in stool or other bleeding)
- No predisposing factors for a bleeding diathesis
Recent field reports suggest that many patients do mount a robust humoral immune response concurrent with reduced viral load but loss of functional integrity of the gut (particularly lower intestine) typically results in bacterial sepsis, shock and death consequent to bacteremia (bacterial infection of the blood). [6] This observation suggests that evolving clinical management strategies may require both novel anti-viral medical countermeasures coupled with more traditional methods for managing both bacterial sepsis from intestinal flora as well as associated shock and sequelae (clinical consequences).
Laboratory testing to confirm the diagnosis is often complicated by challenging field conditions and lack of readily available sophisticated nucleic acid amplification-based capabilities. Ebola viruses may be recovered from soft tissue effusions, semen and anterior eye fluid, especially during later stages of illness. Diagnosis is most typically performed using blood or serum samples. For serological testing, avoid collection tubes with citrate, oxalate or EDTA (Ethylenediaminetetraacetic acid, a chelating agent). For PCR tests, use an EDTA tube, and collect acute-phase specimens within seven days of illness onset. Collect convalescent-phase specimens 7-20 days later and at least 14 days after illness onset. [5] Antigen-capture testing by ELISA (enzyme-linked immunosorbent assay), IgM antibody testing, paired acute-convalescent serum serologies, PCR, immunohistochemistry methods and electron microscopy are advocated but rarely practical options. Cell culture-based viral identification is the reference standard, but the time and hazard associated with this approach restricts use to research and reference labs with high-level biosafety facilities.

U.S. Navy Lt. Jose Garcia pipettes each patient sample into test-ing plate for analysis in order to identify the Ebola virus. Garcia works at a Naval Medical Research Center mobile laboratory at Bushrod Island, Liberia. The NMRC sent two mobile testing labs to Liberia to support Operation United Assistance. Each two-person lab is capable of testing up to 80 samples per day. (U.S. Army Africa photo by Chief Petty Officer Jerrold Diederich/Released)
Methods suitable for field diagnosis of Ebola HF in edge environments are being developed. Evolving pre-published data developed by Rescue Medicine has resulted in development of a practical “five by five” diagnostic algorithm (five clinical signs, five laboratory tests). Current data indicates 92 percent sensitivity and 84 percent specificity relative to PCR-based diagnostic standards. [7] This algorithm is based on a study of 169 prospectively monitored Ebola Bubdybugio and Zaire infected patients being managed using standard of care good clinical practice. In brief, the five by five requires a positive finding for all ten of the following assessment criteria:
Five clinical signs:
Epidemiologic risk (history of contact with an infected individual)
Retro-orbital headache (not meningeal but rather true retro-orbital headache)
Pharyngitis
Myalgia
Documented or subjective fever and/or chills
Five laboratory signs based on field testing with i-STAT or Piccolo point of care laboratory diagnostic devices: [8]
Platelet count less than 100 x 109/L
Transaminitis (increased transaminases (AST, ALT)): at least three-fold increase over normal
AST/ALT ratio of 1.2 to 1.25 to 1
Rising lactate levels (sepsis marker)
Coagulopathy, as measured by whole blood clotting test
Supportive care is essential for patients with Ebola HF and includes maintenance of fluid and electrolyte balance, active hemodynamic monitoring, mechanical ventilation, dialysis and appropriate therapy for secondary infections. Treatment of other suspected causes of disease, such as bacterial sepsis, should not be withheld while awaiting confirmation or exclusion of the diagnosis of Ebola HF. Anticoagulant therapies, aspirin, nonsteroidal anti-inflammatory medications and intramuscular injections are contraindicated. [5]
Current practical considerations for field management in edge environments include obtaining personal protective equipment and rigorous training and reinforcement for all caregivers concerning proper use. Additional recommendations have been developed based on a recent review of 446 critically ill patients with Ebola Bubdybugio and Zaire. [6] Mechanical ventilators and supplies may be useful in some cases (e.g. DARPA SaveII manufactured by AutomedX) but of lower priority relative to colloid, antibiotics and bedside clinical lab units (e.g. i-STAT Abbott point of care testing system). Access to whole blood, PRBC (packed red blood cells) and Leukocyte-depleted platelet preparations is important for clinical management, and platelet infusions have demonstrated statistical improvement in survival during the hemorrhagic phase. Pressor support therapy and supplies (dopamine or other) assist in managing the shock that frequently develops. Fentanyl patches are useful for supportive pain control in field environments. An improvised disposable bedside commode is required to prevent contagion of care providers.
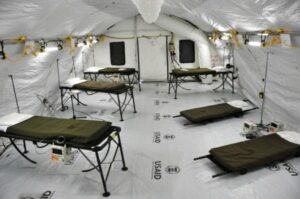
The suspected ward of the Monrovia Medical Unit – an Ebola treatment facility specifically built for medical workers who become infected while caring for patients – stands silent Nov. 4, 2014, before the opening scheduled for Nov. 8, 2014. (U.S. Army photo by Sgt. 1st Class Nathan Hoskins, Joint Forces Command – United Assis-tance Public Affairs/Released)
There are no licensed prophylactic or therapeutic medical countermeasures effective against Ebola virus infection. Current candidate medical countermeasures for Zaire Ebolavirus have demonstrated both preventive and treatment benefit in preclinical testing, but none have yet obtained sufficient clinical trial data to support approval of emergency use authorization by relevant regulatory authorities. However, the severe mortality and morbidity of the present epidemic have already led to their limited use on an emergency, patient by patient basis. Pre-clinical developmental phase vaccines include VSV-vectored recombinants under development by Profectus BioSciences and the Public Health Agency of Canada, and Adenovirus-vectored vaccines under development by multiple firms, and there are non-clinical data suggesting possible utility for both pre- and post-exposure prophylaxis. Potential low molecular weight therapeutics include an RNA i-based product (TKM-Ebola, Tekmira Pharmaceuticals, an interfering ribonucleic acid drug), a nucleoside analog (USAMRIID), a variety of monoclonal antibody preparations including the triEste Fab mAb, which anecdotally may clear viremia without impacting on mortality, [6] and the antisense product AVI-7537 (Sarepta Therapeutics). [9]
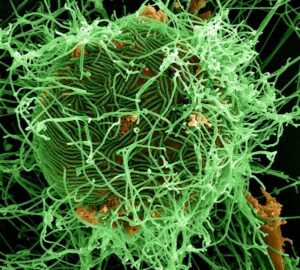
A digitally colorized scanning electron micrograph depicts numerous filamentous Ebola virus particles in green, budding from a chronically infected VERO E6 cell in orange at 25,000X magnification. (Courtesy of National Institute of Allergy and Infectious Diseases/Released)
The CDC documented 12 major Ebola outbreaks between 2000 and the present, with 17 outbreaks of any size (including Reston virus) occurring between 1976 and 2000. [10] Ascel Bio, a leading U.S.-based company focusing on development and application of infectious disease forecasting tools and technology solutions, has analyzed recent outbreaks and is currently actively monitoring and analyzing the current outbreak. [8] This analysis indicates that the level of crisis caused by the current Ebola outbreak is far more severe on a relative basis than the level of crisis caused by any other current disease anywhere else in the world. An Ascel Bio analysis from August 2014 yielded eight key findings: [11]
1. The number of reported human Ebola cases is the highest ever recorded.
The 2014/2015 West Africa Ebola deaths across Guinea, Liberia, Sierra Leone and, previously, Nigeria, are occurring across a very wide geographic scale and have strained international medical response capacity. Ascel Bio assesses case count numbers as the key indicator for success or failure of ongoing or new Ebola containment efforts. Continued high case counts in multiple urban areas with an international Port of Entry increase the likelihood of international translocation.
2. The current case fatality rates (CFR) are within the expected range.
The current CFR for the 2014 West Africa epidemic is 60 percent, against an average CFR of 59 percent across 16 outbreaks over the past twenty years. Historically, Ebola CFRs have been recorded at higher levels. There was a 90 percent CFR observed in Kikwit, Democratic Republic of the Congo (DRC). There is no evidence that the current virus has mutated into something more lethal or more easily transmitted. Furthermore, medical workers appear to be succeeding in keeping the CFRs from rising higher.
3. Ascel Bio has been able to quantitatively confirm that the current outbreak is creating disaster conditions, but there is strong evidence to suggest that the level of disruption caused by the 2014 West Africa Ebola outbreaks is not unique.
Ascel Bio has assessed the level of community disruption caused by Ebola outbreaks across Africa over the past twenty years according to our common standard, the Infectious Disease Impact Scale (or, “IDIS”). The Kikwit, DRC 1994-1995 epidemic, even though it was associated with less case counts than the current epidemic in West Africa, was far more disruptive.
4. There are compelling indications that the causes of the current levels of disruption are unique.
According to Ascel Bio’s analysis, historic causes of disruption that may be ruled out as causes of current disruption include (i.) lack of international recognition and experience of Ebola and (ii.) lack of local recognition and experience of Ebola. Restated another way, the problem today is not that people do not understand that they are facing Ebola, which was a condition that had led to maximal, sustained disruption in the past. It is Ascel Bio’s assessment that risk communication and education campaigns that should be driving containment have been ineffective, if not counterproductive. The level of disruption is rooted in locals’ rejection of these initiatives associated with international aid workers. These conditions are similar to what was observed by Ascel Bio after the 2010 Haiti earthquake. It is Ascel Bio’s assessment that the broad solutions will require a specific focus on fixing communications and trust failures at the local level.
5. There are indications of drivers for evacuation and flight.
It is important to recognize that evacuation and flight are common, routine components of the Ebola signature pattern. In the case of Sierra Leone and Liberia, non-essential personnel have been evacuated; however, current reporting suggests expatriate healthcare workers will seek to evade airport screening procedures and violate their quarantine contract in an effort to flee the epidemic zones. At the community level, we have indication of non-ambiguous disaster conditions, as mentioned above. Ascel Bio has received multiple reports of abandonment of the dead in indigenous homes, where the inhabitants fled in fear. This implies a potential to reach an extremely rare, but known “apocalyptic” level of disruption referred to as an IDIS CAT 7. This level of disruption refers to total loss of social control to the point of abandonment of living family members, also known as familial disintegration. This has been observed rarely in history, and has been seen with First Contact situations with Ebola, Marburg and Nipah viruses. The importance of this is a community shift from convergence to care for loved ones to divergence away from the ill. The latter point then highlights the threat of further expansion throughout the region. Therefore, we continue to have risk of both terrestrial and airflight-based translocation of the epidemic.
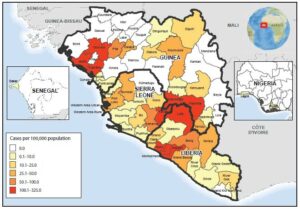
The map of West Africa shows Ebola virus disease cumulative incidence as of September 20, 2014. Geo-graphic distribution of the cumulative incidence of Ebola, as of September 23, indicates that the highest cumulative incidence (>100 cases per 100,000 population) was found in five districts in Guinea (Boffa, Dubreka, Gueckedou, Macenta, and Telimele), two districts in Liberia (Loffa and Margibi) and two districts in Sierra Leone (Kailahun and Kenema). (Courtesy of http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6339a4.htm/Released)
6. Regarding conflict, both Sierra Leone and Liberia continue to exhibit strong social unrest signature patterns.
Social unrest is also a common signature component of Ebola to the point of presenting a threat to in-country international responders, as noted in the current situation. State failure has not been documented to date, and Ascel Bio does not assess this to be a probable (albeit not impossible) outcome.
7. Regarding translocation to developed nations such as the United States, Ascel Bio does not assess there to be a significant concern in terms of seeing an uncontrolled epidemic.
Small case clusters are possible. The key to mitigation of the potential for IDIS CAT 3/crisis conditions is the element of surprise: local physicians must be sensitized to the possibility of translocation and prepared through education and preparedness planning. Particular areas of sensitivity include the emergency department and intensive care units, which are critical structures that ensure continued ingestion of patients for any developed nation’s hospitals. Live feedback from one of the nation’s largest social networks for physicians, Sermo, has indicated significant anxiety and concern that the medical community is unprepared:
How prepared do you feel the US healthcare system is to respond to an Ebola outbreak?
• Very unprepared (383/929)
• Extremely unprepared (315/929)
• Neither prepared or unprepared (157/929)
• Very prepared (65/929)
• Extremely prepared (9/929)
8. One Ascel Bio team member was in the process of prepping to deploy in with Samaritan’s Purse to support medical response. His observations about federal assistance associated with this potential deployment include the following:
- Finding a single focal point for credible, vetted information was difficult, which is typical in a disaster response situation.
- There was significant difficulty obtaining the latest guidance for Personal Protection Equipment (PPEs), clinical management protocols and specific isolation ward management information. Many SMEs in the government had differing views, where disagreements between agencies resulted in lack of clear
- Consensus regarding therapeutics. The impression that the Ascel Bio team member is the U.S. government no longer enjoys the same level of experience in Ebola management it once had in the late 1990s.
- Protocols were not in place beforehand to assist non-U.S. government American citizens in medical evacuation and aggressive treatment for high consequence pathogens.
Based on this analysis and observations, the following scenario probabilities for the current outbreak have been assigned by Ascel Bio operational biosurveillance subject matter experts based on a review of available data.
Scenario 1: Locals will learn & adopt effective containment practices in the next weeks, sui generis.
| Probability | Scenario |
| 20% | Fatality numbers alone will create conditions where locals generate independent reasons to apply the same containment methods being promoted by international health authorities that they are currently rejecting. |
| 80% | Absent a resolution of ineffective risk communication and education campaign approaches, the level of disruption will remain high, and medical countermeasures will continue to be sub-optimal.
|

Members of a joint service medical support team don their Protective Personal Equipment as part of their training for personnel to respond quickly, effectively and safely in the event of additional Ebola cases in the United States, on San Antonio Military Medical Center, Texas, Oct. 23, 2014. (U.S. Air Force photo by Airman 1st Class David R. Cooper/Released)
Scenario 2: Locals will learn and adopt effective containment practices in the next weeks through a resolution of current trust issues.
| Probability | Scenario |
| 40% | Through concerted efforts to better engage directly with local community leaders, trust will be built to enable effective containment practices. |
| 60% | Locals will remain hardened against international health workers in the weeks ahead. |
Based on this most recent analysis, Ascel Bio concludes that “High fatality numbers, spread, and continued hardening against international health workers, coupled with a strained medical infrastructure, from international to local capabilities, across multiple urban areas with international Ports of Entry would create conditions suitable to more translocation and threaten far higher fatalities.”[11] To further illustrate the potential risks associated with this current outbreak, public health experts have expressed concerns about scenarios that may present direct threats to the continental United States (CONUS). One current example of a CONUS safety concern involves expatriate healthcare provider elopement from quarantine. Twelve of the forty nine United States expatriate healthcare providers working in West Africa supporting the international medical response to the current outbreak have violated the terms of their quarantine contract and returned prematurely to CONUS.[6]
As the epidemic expands in and beyond West Africa, and international efforts surge to provide new medical countermeasures, National Health Authorities and local practitioners will be faced with the dilemma as to whether to use a potentially growing number of medical countermeasures provided under an emergency or investigational new drug application framework. These decisions will also be taken in the context of rampant local mistrust of medical quarantine and hospitalization practices, which have already led to treatment avoidance, hospital flight and violence against health care workers. For this reason, there is a growing need for Host Nations, International Health Authorities (WHO) and medical countermeasure (MCM) donor nations to develop a practical ethical framework for triaging the use of MCMs to provide the greatest possible public health benefit and to manage expectations for their availability and safe use.

Supporting Operation United Assistance, Armed Forces of Liberia engineers work on the Tubmanburg Ebola treatment unit. Crews quickly put up a series of tents on freshly leveled and graveled ground. The U.S. Agency for International Development is the lead U.S. Government organization for Operation United Assistance. U.S. Africa Command is supporting the effort by providing command and control, logistics, training and engineering assets to contain the Ebola virus outbreak in West African nations. (U.S. Army Africa photo /Released)
References:
[1] “Ebola hemorrhagic fever.” 22 Jan. 2008, University of Maryland Medical Center. 6 Dec. 2008, http://www.umm.edu/ency/article/001339trt.htm.
[2] “Ebola Hemorrhagic Fever” CDC website, accessed 31 July 2014, http://www.cdc.gov/vhf/ebola/.
[3] “Ebola virus disease” WHO Fact sheet N°103, Updated April 2014, http://www.who.int/mediacentre/factsheets/fs103/en/.
[4] “African Haemmorhagic Fever In The Southern Sudan, 1976: The Clinical Manifestations” D.H. Smith, D.P. Francis, D.I.H. Simpson. Website accessed 31 July 2014.
[5] S.F. Dept. Public Health – Infectious Disease Emergencies, VIRAL HEMORRHAGIC FEVER, August 2005.
[6] Michael Callahan, MD, Massachusetts General Hospital, Boston, MA. Manuscript in Preparation.
[7] Travel Medicine (Second Edition), Mosby/Elsevier Ltd. Chapter 47 – Bites, Stings, and Envenoming Injuries, p 463-473. Michael Callahan, author; JS Keystone et al., Editors.
[8] “Ebola drugs still stuck in lab.” M. Enserink, Science 25 July 2014, Vol 345, Issue 6195, p 364-365.
[9] “Outbreak Postings” CDC website accessed 31 July 2014, http://www.cdc.gov/vhf/ebola/resources/outbreaks.html.
[10] James M. Wilson V, MD. Personal communication.
About the author:
Dr. Robert W. Malone, MD, MS, served as a subject matter expert to the HDIAC Basic Center Operations (BCO) support team at TASC Inc. A Maryland-licensed physician trained in molecular virology and immunology at The Salk Institute Molecular Biology and Virology Laboratories, and in Clinical and Anatomic Pathology at UC Davis Sacramento Medical Center, Dr. Malone is an original (1989) inventor of “naked DNA” and “DNA Vaccine” technology. Dr. Malone is also recognized for pioneering scientific contributions in RNA delivery and non-viral gene transfer (cationic lipids, in-vivo electroporation). Since the unfortunate events surrounding 911, Dr. Malone has focused on facilitating pre-clinical to clinical transition and advanced development of medical countermeasures to biothreat agents in support of DoD and HHS missions, with his current focus being on supporting the TASC CSIS Medical division.