Nerve agents are toxic organophosphates that disrupt chemical signaling throughout the central and peripheral nervous systems. The structures of several common nerve agents are shown in Figure 1. These compounds have been used sparingly throughout history, in accordance with the Geneva Protocol of 1925. However, there have been bad actors and brazen violations. Notable instances include the 1980-1988 Iraq-Iran War, the Tokyo Subway sarin attack of 1995, the Ghouta sarin attack of 2013, and the most recent Khan Shaykhun chemical attack of 2017 where the Syrian government poisoned its own civilians with sarin [1-3]. These incidents, coupled with the rise of radical terrorist groups like the Islamic State, have prompted scientists to improve antidotes for nerve agents as the threat of a chemical attack increases both at home and overseas. Such research is of considerable interest to the U.S Department of Defense (DoD). Herein, we provide an overview on the biology and chemistry of nerve agents, reveal the limitations of current treatments, and highlight key studies that pave the way toward second-generation therapeutics.
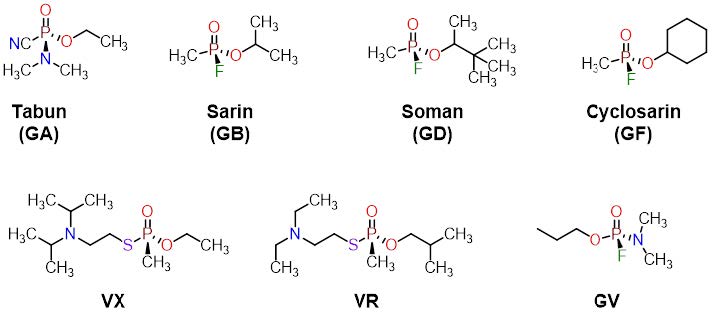
Figure 1. Structures of selected nerve agents used in chemical warfare
Chemical Properties
Nerve agents are generally odorless, colorless, and tasteless liquids with high boiling points and low volatility. The term “nerve gas” is thus a misnomer and stems from the fact that nerve agents are aerosolized to the gaseous state upon deployment for rapid delivery to the respiratory system.
With respect to absorption, VX and VR are relatively lipophilic and are readily absorbed through the skin, whereas smaller compounds, like sarin, are more volatile and have hydrophilic character that allows deeper penetration into the lungs [4]. Toxicity is a function of the nerve agent, exposure time, and route of delivery with symptoms appearing anywhere from 30 seconds to 18 hours following exposure [5].
Symptoms vary from mild to severe and include visual impairment, nausea, muscle spasms (fasciculation), respiratory failure, unconsciousness, and death. Dermal exposure to VX is readily countered by the generous application of Reactive Skin Decontamination Lotion (RSDL) on unbroken skin [6]. RSDL is the DoD’s preferred countermeasure for cutaneous exposure of VX, however, emerging research from the U.S. Army Medical Research Institute of Chemical Defense has identified a new topical antimicrobial anti-infective/disinfectant, Veriox®, that is 1.8 times more effective than RSDL at neutralizing VX in animal survival/ lethality models [7].
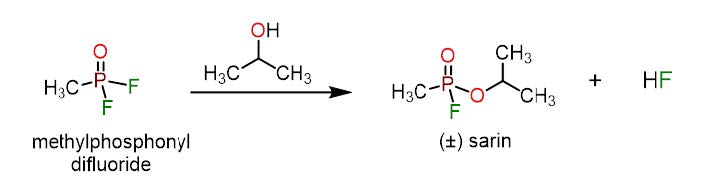
Figure 2. The synthesis of sarin is readily accomplished from cheap precursors methylphosphonyl difluoride and isopropanol. Hydrofluoric acid is produced as a byproduct.
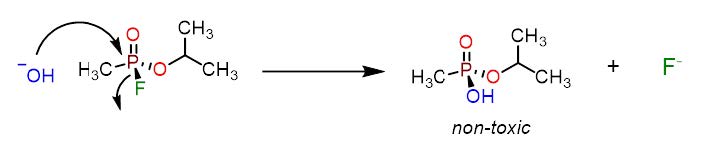
Figure 3. The inactivation of sarin by hydroxide ions produces a non-toxic phosphonic acid and fluoride ions
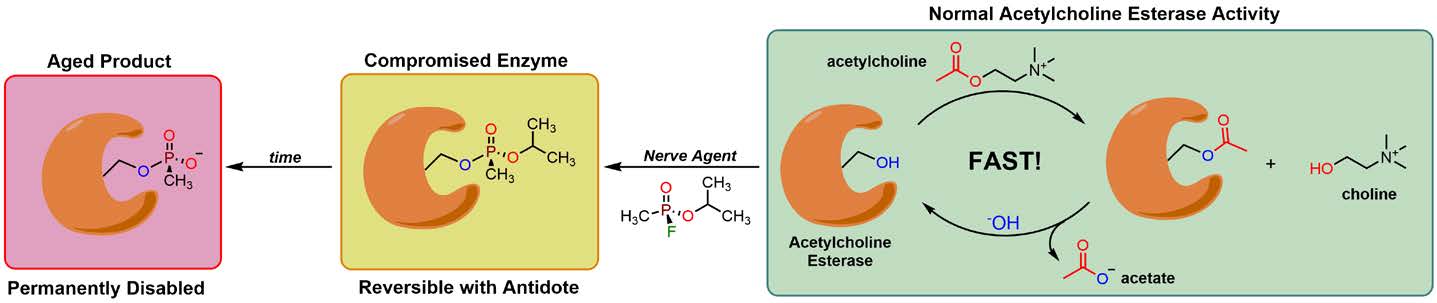
Figure 4. The mechanism of AChE inactivation by sarin. Green block: Normal AChE activity is characterized by the rapid turnover of AChE to produce choline and acetate at the synaptic cleft. Orange block: Covalent modification of Ser203 in the active site of AChE compromises the enzyme’s activity and causes a build-up of acetylcholine at the synapse. Oxime antidotes can reverse this modification if supplied promptly after exposure. Red block: As time progresses, the nerve agent becomes dealkylated to form an aged product that permanently disables AChE. Recovery with an antidote is not possible at this stage.
In contrast to many biological toxins, such as tetrodotoxin (pufferfish poison), batrachotoxin (poison dart frogs), botulinum toxin, and ricin, phosphorous-derived nerve agents can be easily prepared in large quantities from cheap and readily available precursors. This makes nerve agents the preferred class of chemical weapons for nation-states and terrorist groups. Sarin is prepared in a single step from the reaction between methylphosphonyl difluoride and isopropanol (Figure 2). Cyclosarin and soman are accessed in a similar fashion by substituting isopropanol with cyclohexanol and pinacoyl alcohol, respectively. In binary weapons, the precursors are separated in two chambers that break upon deployment to produce the nerve agent in situ. This significantly reduces the risk of premature exposure, accidental dispersion, and improves transportability and storage [8].
With respect to their chemical reactivity, all the nerve agents shown in Figure 1 feature a central phosphorous atom flanked with different substituents. These organophosphates share a similar structural motif that is responsible for their high reactivity and inherent toxicity—a leaving group.
A leaving group is an atom or molecular fragment that departs from a target molecule and is substituted by an incoming nucleophile. For soman, sarin, and cyclosarin, fluoride (F- ) is the designated leaving group and is readily displaced from the phosphorous center by oxygen nucleophiles. The facile displacement of fluoride by oxygen-containing molecules can be exploited for large scale chemical decontamination as shown in Figure 3. Alkaline solutions of sodium hydroxide dissolved in methanol or ethanol readily decontaminates most nerve agents, but these solutions are caustic and alternative formulations have been developed. One such formulation is DF200, a non-corrosive and biodegradable aqueous foam that contains alkaline hydrogen peroxide as the decontaminating agent [9]. The expanding foam maximizes contact with contaminated surfaces and provides a visual for treated areas. DF200 is highly effective against tabun, sarin, soman, and VX.
Mechanism of Action
Nerve agents exert their toxic effects in humans, insects, and other organisms by inhibiting the enzyme acetylcholine esterase (AChE). AChE hydrolyzes the neurotransmitter acetylcholine into choline and acetate, thereby preventing the accumulation of acetylcholine at the synaptic cleft. The near instantaneous degradation of acetylcholine by AChE terminates the synaptic signal and allows the nicotinic and muscarinic receptors at the neurological synapse to momentarily relax before incoming acetylcholine molecules bind them again. This excitation-relaxation process facilitates an uncluttered stream of chemical signals that is essential for nerve impulses and muscle innervation. Because of this, AChE is prominently expressed throughout the peripheral and central nervous systems.
AChE features a catalytic serine residue in the active site that is responsible for the enzyme’s hydrolytic activity. Serine possesses an oxygenic side chain that is vulnerable to irreversible covalent modification by organophosphates. Figure 4 illustrates the stages of AChE inactivation by sarin. The right side of Figure 4 shows the rapid turnover of AChE and the hydrolysis of acetylcholine with the catalytic serine shown in the pocket of the enzyme. Serine displaces the leaving group on the nerve agent to form a covalent adduct between AChE, compromising the enzyme (see Figure 4, orange block). As time passes, the nerve agent-AChE enzyme complex undergoes a series of intimate conformational changes that dealkylate the nerve agent [10]. This event generates an “aged product” (see Figure 4, red block) and permanently disables AChE. At this stage, the enzyme cannot be salvaged by the administration of an antidote.
The inactivation of a single AChE molecule is not deleterious. However, as more and more AChE becomes compromised, excess acetylcholine begins to develop at the neurological synapse resulting in unrelenting excitation of nicotinic and muscarinic receptors located throughout the peripheral and central nervous systems. The buildup of acetylcholine and uncontrollable excitation creates a cholinergic crisis characterized by salivation, cognitive impairment, convulsions, bronchoconstriction, fasciculation and paralysis, culminating in death caused by a cascade of seizures and/or respiratory failure [11].
Available Treatments
The mainstay combination treatment strategy for nerve agent poisoning includes atropine sulfate, diazepam, and pralidoxime (2-PAM), with each having a different mechanism of action and purpose in treatment (see Figure 5, first row). These compounds are formulated into various Antidote Treatment Nerve Agent Autoinjector (ATNAA) kits used by the U.S. military and serve as the first line of defense against nerve agents for soldiers on the battlefield (see Figure 6). Atropine is a muscarinic antagonist and is administered to competitively block the effects of excess acetylcholine at the synaptic cleft. Atropine partially mitigates the physiological effects of a mounting cholinergic crisis at muscarinic receptors. However, it does not antagonize nicotinic receptors located on somatic neurons which are also overstimulated by excess acetylcholine. Continuous nicotinic receptor signaling produces severe physiological consequences including paralysis and convulsions. Unfortunately, there have been few attempts to investigate competitive nicotinic receptor antagonists primarily due to the difficulty in achieving a dose of compound sufficient to confer protection against acetylcholine overstimulation without inducing paralysis [4].
The deficits associated with atropine have led to the incorporation of diazepam (see Figure 5) in modern day treatments. Diazepam is a positive allosteric modulator of the GABAA receptor and effectively counters the onset of seizures arising from the overstimulation of nicotinic receptors in the amygdala [12, 13]. However, as with atropine, diazepam is not a true antidote. Neither drug restores the catalytic activity of AChE. This feat is accomplished by another class of compounds known as oximes. Oximes dislodge the nerve agent from the active site of the enzyme to regenerate free AChE where it can then properly hydrolyze acetylcholine.
The hallmark of the oxime family is pralidoxime (2-PAM, see Figure 5) and its congeners: obidoxime and trimedoxime. The combination of atropine and 2-PAM act synergistically to raise the LD50 of nerve agents by a factor of twenty [14]. However, the efficacy of ATNAA kits is highly contingent on the identity of the nerve agent and the time at which the antidote is administered due to adduct aging (see Figure 4, red block). As previously mentioned, aged adducts cannot be rescued by oxime antidotes. The aging process is highly variable between nerve agents and accounts for dramatic differences in treatability. For example, soman adducts age in less than fifteen minutes whereas VR adducts age in roughly 139 hours [4]. Note that the reaction time of the ATNAA kit is approximately ten to fifteen minutes. Consequently, soman is a formidable poison on the battlefield and is exceptionally difficult to treat in real-world situations [4]. To combat fast-aging nerve agents like soman, military personnel at high risk of exposure may be supplied with pyridostigmine, a reversible AChE inhibitor (see Figure 5). Pyridostigmine reversibly binds the AChE active site thereby reducing the likelihood of covalent modification by nerve agents [15]. After treatment, pyridostigmine dissociates from the enzyme to facilitate unabated AChE activity.
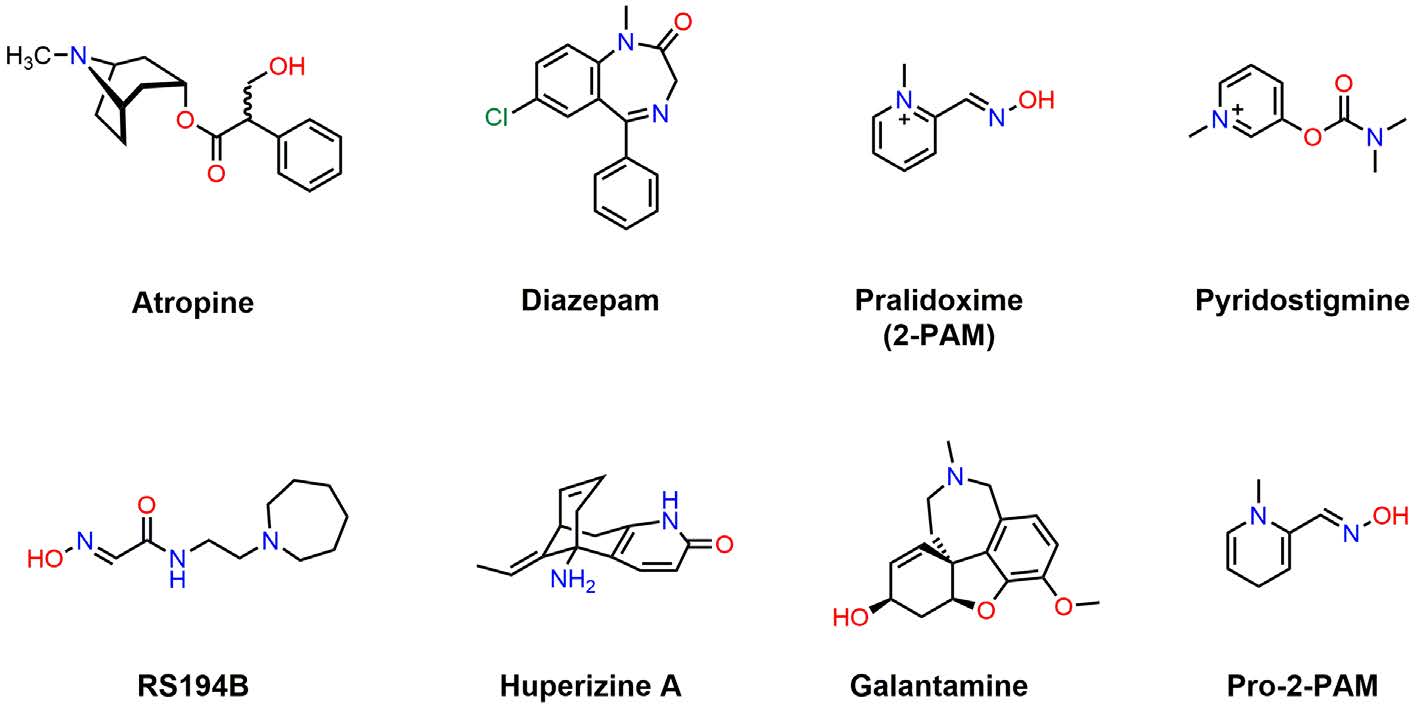
Figure 4. The mechanism of AChE inactivation by sarin. Green block: Normal AChE activity is characterized by the rapid turnover of AChE to produce choline and acetate at the synaptic cleft. Orange block: Covalent modification of Ser203 in the active site of AChE compromises the enzyme’s activity and causes a build-up of acetylcholine at the synapse. Oxime antidotes can reverse this modification if supplied promptly after exposure. Red block: As time progresses, the nerve agent becomes dealkylated to form an aged product that permanently disables AChE. Recovery with an antidote is not possible at this stage.
There are several limitations associated with the therapies described above. First, pyridostigmine and 2-PAM feature a permanent cationic charge that restricts access to the central nervous system (CNS). Second, atropine antagonizes muscarinic but not nicotinic receptors at the synaptic cleft [16]. Third, 2-PAM is not a broad-spectrum antidote and has limited efficacy against cyclosarin, soman, and tabun [17]. Finally, no antidote can reactivate inhibited AChE once it ages. These shortcomings have prompted researchers and the DoD to investigate novel therapies to confer better protection against nerve agents for troops stationed abroad.
Emerging Treatment Strategies
The reactivation of AChE in the CNS is essential to reduce lethality following exposure to nerve agents. One of the key challenges associated with 2-PAM is its limited ability to reactivate AChE in the CNS. To address this problem, a series of nonquaternary oximes have been developed [18]. One of the most promising of these is Pro-2-PAM, shown in Figure 5. Pro-2-PAM does not feature a permanent cationic charge and is metabolized to 2-PAM in neurons. This structural change should permit translocation of the free base across the blood brain barrier and access to the CNS. In 2010, Gordon and collaborators put that hypothesis to the test [19]. The researchers exposed guinea pigs to lethal doses of diisopropylfluorophosphate and subsequently treated the animals with either pro-2-PAM or 2-PAM (13 mg/kg). The authors note that pro-2-PAM considerably reduced the onset of seizures and neurological damage when compared to 2-PAM, thereby confirming the enhanced efficacy of pro-2-PAM in an animal model of organophosphate exposure [19].
RS194B is another promising oxime antidote that has garnered significant attention in recent years. Discovered by Nobel Laureate Barry Sharpless and Palmer Taylor, RS194B demonstrates significant CNS permeability and AChE reactivation in organophosphate exposed mice [20]. In addition, RS194B boasts an attractive pharmacokinetic profile and has been recently shown to reverse the clinical symptoms of sarin inhalation in non-human primates after a single IM injection (62.5 mg/kg) [21]. These results suggest that RS194B is an exciting candidate with real clinical potential to improve existing organophosphate antidote regimens.

Figure 6. Antidote Treatment Nerve Agent Autoinjector (ATNAA) containing atropine and pralidoxime chloride used by the U.S. military
The reversible AChE inhibitor galantamine has emerged as an attractive alternative to pyridostigmine when administered before or promptly after exposure to a nerve agent [22]. Galantamine demonstrates good CNS penetration and protects guinea pigs from soman-induced brain damage when administered (at 8 mg/kg via IM) thirty minutes prior to exposure [23, 24]. Interestingly, galantamine is Food and Drug Administration (FDA) approved for the treatment of Alzheimer’s disease. The DoD has investigated the use of neuroprotective peptides to complement small molecules like galantamine [25].
Huperizine A is another reversible AChE inhibitor that reaches the CNS. Unlike galantamine and pyridostigmine, Huperizine A is not FDA approved. However, it is approximately eighty-eight times more potent than galantamine, touts a superior safety profile, and prevents soman toxicity in cynomolgus macaques when administered as a pretreatment [26]. If future trials return positive results, Huperizine A may become the preferred pretreatment option for troops in the future.
To block nicotinic receptor signaling during a cholinergic crisis, non-competitive antagonists have been investigated. Non-competitive antagonists do not compete with acetylcholine at the receptor, thus eliminating the dosing problem associated with competitive antagonism described previously. This area has been met with some success and a series of bispyridinium compounds have been identified that block nicotinic acetylcholine signaling in guinea pigs and human cell lines [16, 27].
When co-administered with atropine, the non-competitive nicotinic antagonist 1,1′-(propane-1,3-diyl)bis(4-tert-butylpyridinium) was shown to reverse neuromuscular paralysis in guinea pigs exposed to sarin and tabun [16]. Additional studies are needed to evaluate both the safety and efficacy of this compound in human subjects.
Conclusion
In summary, nerve agents remain a challenging adversary for troops stationed overseas. The limitations associated with current treatments have led to the development of CNS-penetrant antidotes like Pro-2-PAM and RS194B. Huperizine A and galantamine are investigative pretreatment options that demonstrate enhanced CNS permeability over pyridostigmine and are better suited to protect soldiers in the event of a nerve agent attack. Nicotinic receptor antagonists complement muscarinic receptor antagonists and successfully mitigate the toxicity acetylcholine during a nerve agent induced cholinergic crisis. These research efforts highlight the significant progress that has been made towards improving antidotes for military personnel and provide a strong foundation for the development of next-generation therapeutics to combat organophosphate nerve agents on the battlefield.
References
1. Pita, R., & Domingo, J. (2014). The use of chemical weapons in the Syrian conflict. Toxics, 2(3), 391-402.
2. Byers, M. (2014). Deliberate chemical attack: revisiting the lessons of the Tokyo subway attack. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, 22(Suppl 1), A8- A8. doi:10.1186/1757-7241-22-S1-A8
3. Brooks, J., Erickson, T. B., Kayden, S., Ruiz, R., Wilkinson, S., & Burkle, F. M., Jr. (2018). Responding to chemical weapons violations in Syria: Legal, health, and humanitarian recommendations. Conflict and Health, 12, 12-12. doi:10.1186/s13031-018-0143-3
4. Costanzi, S., Machado, J. H., & Mitchell, M. (2018). Nerve Agents: What They Are, How They Work, How to Counter Them. ACS Chemical Neuroscience, 9(5), 873-885. doi:10.1021/acschemneuro.8b00148
5. Wiener, S. W., & Hoffman, R. S. (2004). Nerve agents: a comprehensive review. Journal of Intensive Care Medicine, 19(1), 22-37. doi:10.1177/0885066603258659
6. Thors, L., Lindberg, S., Johansson, S., Koch, B., Koch, M., Hagglund, L., & Bucht, A. (2017). RSDL decontamination of human skin contaminated with the nerve agent VX. Toxicology Letters, 269, 47-54. doi:10.1016/j.toxlet.2017.02.001
7. Koplovitz, I. S., Susan; Morgan, Julia; Rousayne, Cassandra; Clarkson, Edward. (2016). Evaluation of Veriox as a Skin Decontamination Product after Dermal Exposure to the Nerve Agent VX. US Army Medical Research Institute of Chemical Defense.
8. Pitschmann, V. (2014). Overall view of chemical and biochemical weapons. Toxins, 6(6), 1761-1784. doi:10.3390/toxins6061761
9. Khan, A. W., Kotta, S., Ansari, S. H., Ali, J., & Sharma, R. K. (2013). Recent advances in decontamination of chemical warfare agents. Defence Science Journal, 63(5), 487-496.
10.Saxena, A., Doctor, B. P., Maxwell, D. M., Lenz, D. E., Radic, Z., & Taylor, P. (1993). The role of glutamate-199 in the aging of cholinesterase. Biochemical and Biophysical Research Communications, 197(1), 343-349. doi:10.1006/bbrc.1993.2481
11. King, A. M., & Aaron, C. K. (2015). Organophosphate and carbamate poisoning. Emergency Medicine Clinics, 33(1), 133-151.
12.Moshiri, M., Darchini-Maragheh, E., & Balali-Mood, M. (2012). Advances in toxicology and medical treatment of chemical warfare nerve agents. Daru, 20(1), 81. doi:10.1186/2008-2231-20-81
13.Iha, H. A., Kunisawa, N., Shimizu, S., Tokudome, K., Mukai, T., Kinboshi, M., . . . Ohno, Y. (2017). Nicotine Elicits Convulsive Seizures by Activating Amygdalar Neurons. Frontiers in Pharmacology, 8, 57-57. doi:10.3389/fphar.2017.00057
14.Eddleston, M., Buckley, N. A., Eyer, P., & Dawson, A. H. (2008). Management of acute organophosphorus pesticide poisoning. Lancet (London, England), 371(9612), 597-607. doi:10.1016/S0140-6736(07)61202-1
15.Keeler, J. R., Hurst, C. G., & Dunn, M. A. (1991). Pyridostigmine used as a nerve agent pretreatment under wartime conditions. JAMA, 266(5), 693-695.
16.Turner, S. R., Chad, J. E., Price, M., Timperley, C. M., Bird, M., Green, A. C., & Tattersall, J. E. H. (2011). Protection against nerve agent poisoning by a noncompetitive nicotinic antagonist. Toxicology Letters, 206(1), 105-111. doi:10.1016/j.toxlet.2011.05.1035
17.Romano, J. A., Lukey, B. J., & Salem, H. (2008). Chemical warfare agents: Chemistry, pharmacology, toxicology, and therapeutics (2nd ed.). Boca Raton: CRC Press.
18.Wei, Z., Liu, Y.-q., Wang, Y.-a., Li, W.- h., Zhou, X.-b., Zhao, J., . . . Li, S. (2016). Novel nonquaternary reactivators showing reactivation efficiency for soman-inhibited human acetylcholinesterase. Toxicology Letters, 246, 1-6. doi:10.1016/j.toxlet.2016.01.015
19.DeMar, J. C., Clarkson, E. D., Ratcliffe, R. H., Campbell, A. J., Thangavelu, S. G., Herdman, C. A., . . . Gordon, R. K. (2010). Pro-2-PAM therapy for central and peripheral cholinesterases. Chemico-Biological Interactions, 187(1), 191- 198. doi:10.1016/j.cbi.2010.02.015
20.Radić, Z., Sit, R. K., Kovarik, Z., Berend, S., Garcia, E., Zhang, L., . . . Taylor, P. (2012). Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases. Journal of Biological Chemistry, 287(15), 11798- 11809. doi:10.1074/jbc.M111.333732
21.Rosenberg, Y. J., Mao, L., Jiang, X., Lees, J., Zhang, L., Radic, Z., & Taylor, P. (2017). Post-exposure treatment with the oxime RS194B rapidly reverses early and advanced symptoms in macaques exposed to sarin vapor. Chemico-Biological Interactions, 274, 50-57. doi:10.1016/j.cbi.2017.07.003
22.Golime, R., Palit, M., Acharya, J., & Dubey, D. K. (2018). Neuroprotective effects of galantamine on nerve agent-induced neuroglial and biochemical changes. Neurotoxicity Research, 33(4), 738-748. doi:10.1007/s12640-017-9815-9
23.Alexandrova, E. A., Aracava, Y., Pereira, E. F., & Albuquerque, E. X. (2010). Pretreatment of guinea pigs with galantamine prevents immediate and delayed effects of soman on inhibitory synaptic transmission in the hippocampus. Journal of Pharmacology and Experimental Therapeutics, 334(3), 1051-1058. doi:10.1124/jpet.110.167700
24.Gullapalli, R. P., Aracava, Y., Zhuo, J., Helal Neto, E., Wang, J., Makris, G., . . . Albuquerque, E. X. (2010). Magnetic resonance imaging reveals that galantamine prevents structural brain damage induced by an acute exposure of guinea pigs to soman. Neurotoxicology, 31(1), 67-76. doi:10.1016/j.neuro.2009.09.004
25.New approach for neuroprotection against nerve agents: A novel peptide ameliorates neuroinflammation. (2016). Defense Threat Reduction Agency.
26.Hamilton, L. R., Schachter, S. C., & Myers, T. M. (2017). Time course, behavioral safety, and protective efficacy of centrally active reversible acetylcholinesterase inhibitors in cynomolgus macaques. Neurochemical Research, 42(7), 1962-1971. doi:10.1007/s11064-016-2120-9
27.Ring, A., Strom, B. O., Turner, S. R., Timperley, C. M., Bird, M., Green, A. C., . . . Tattersall, J. E. H. (2015). Bispyridinium compounds inhibit both muscle and neuronal nicotinic acetylcholine receptors in human cell lines. PloS One, 10(8), e0135811. doi:10.1371/journal. pone.0135811


