Modern aircraft and ground vehicles rely on traditional power and energy storage devices. Due to their high mass and volume requirements, these systems can limit the effectiveness and range of Department of Defense (DoD) vehicles and other assets. Structural energy and power systems—those that act as both the structural support and the energy storage device through the use of multifunctional materials—offer a unique strategy for reducing the mass or volume of traditional systems by simultaneously managing energy storage and mechanical stress. The concept centers on combining the performance of structural composites with batteries and supercapacitors [1–3]. This bears specific relevance to DoD in that structural energy storage could reduce mass in cube satellites and aircraft, for example, allowing for the maximization of propulsion resources (see Figure 1a).
Additionally, mechanically robust supercapacitors and batteries may be able to resist damage from micrometeoroids and orbital debris, high-velocity ballistic impacts, unexpected collisions, and explosions—providing enhanced safety for vehicles and their occupants. The U.S. Army Research Laboratory has long been interested in developing structural composites with battery functionality to improve battery efficiency while reducing weight and volume [4]. Furthermore, when fabricated to the form factor of body armor, structural energy and power systems may provide the warfighter with ballistic protection and electrical power simultaneously. Such a technology would be integral in aiding the development of the future Special Forces hyper-enabled operator [5].
However, the major challenge to developing structural energy materials is that an inherent trade-off exists between mechanical and electrochemical performance [1]. For example, improvements in mechanical properties come at the cost of losses in electrochemical performance, and vice versa. This article first provides a brief overview of materials for structural energy and power, as well as a discussion of the metrics used to describe this multifunctional concept. Then, we will focus specifically on the use of reduced graphene oxide nanosheets and Kevlar nanofibers as key enablers of novel structural energy and power systems
Background
Batteries and supercapacitors, two common devices used to provide energy and power, are comprised of two electrodes, an electrolyte, and a separator (see Figures 1b–c). The electrodes allow redox reactions to occur in batteries or to create electrostatic charge separation in supercapacitors. The electrolyte facilitates ion transport between the electrodes, and the separator prevents direct electrical contact between the two electrodes—an important component of battery safety. Typical battery electrode materials, such as lithium cobalt oxide (LiCoO2 ), lithium manganese oxide (LiMn2 O4 ), and lithium iron phosphate (LiFePO4 ), are poor candidates for structural electrodes due to their brittle nature. Carbon aerogel, a common supercapacitor electrode material, also possesses poor mechanical properties, having a strength of 0.15 MPa and a Young’s modulus of 2.8 MPa [7]. Poly(ethylene oxide)-based solid electrolytes have low mechanical properties (Young’s modulus of 0.36 GPa) [8]. Common separators also have low moduli; for example, poly(propylene) separators have a Young’s modulus of only 430 MPa and a strength of 14.2 MPa [9]. Clearly, typical energy storage materials do not possess the sufficient mechanical properties required for structural energy and power.
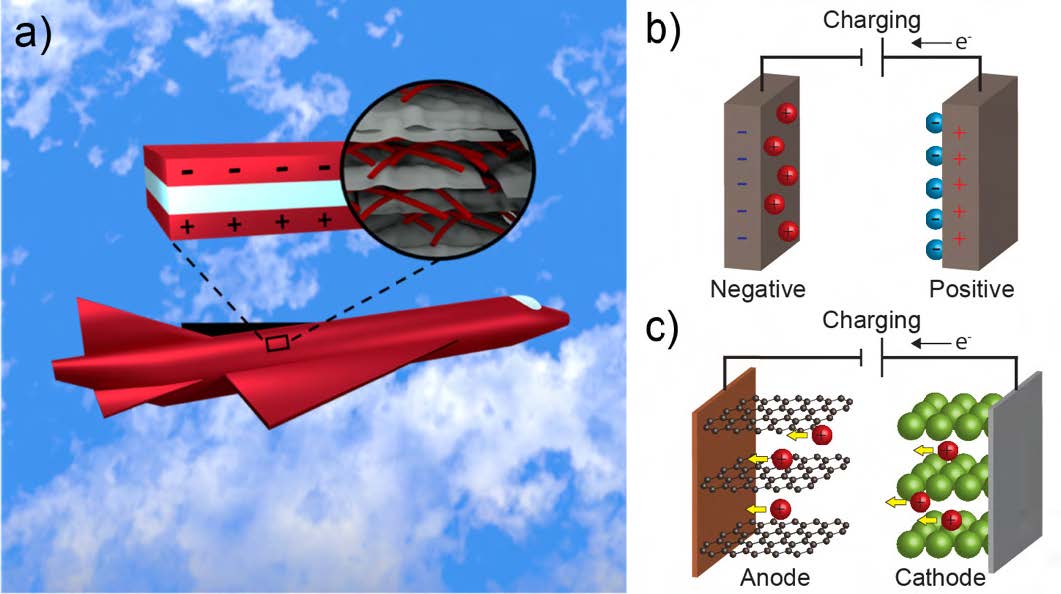
Figure 1. a) Conceptual design for structural energy and power systems as outer paneling of a plane [6] and schematic representations of a b) battery and c) supercapacitor
Previous work on structural energy storage by the Wetzel [1, 2], Greenhalgh [3], Pint [10], and Asp [11] groups has focused on using carbon fibers as a multifunctional electrode material. These electrodes typically possess good mechanical properties (Young’s modulus of 41 GPa), but have exhibited a low capacitance of 93 mF g-1 [2]. Carbon nanotube-based structural electrodes produced by the Pint group faced a similar trend, as the Young’s modulus was 6.2 GPa but the capacitance was 16 mF g-1 [10]. These previous studies prioritized mechanical performance over energy storage. The aforementioned carbon-based materials have exhibited good mechanical properties thus far, but improvements in energy storage performance are still needed.
While much attention has been placed on structural electrodes, structural electrolyte/ separator reports by the Lutkenhaus [12], Veith [13], Yang [14], and Shaffer [15] groups have described promising materials that conduct ions and withstand mechanical forces or impact. For example, the Lutkenhaus group developed a shear-thickening electrolyte consisting of anisotropic silica nanorods [12], and the Veith group used nonfumed, monodisperse silica [13]. Yang demonstrated poly(vinylidene fluoride) and palygorskite ((Mg,Al)2 Si4 O10(OH)) nanowire composites that showed a Young’s modulus of 96 MPa and strength of 1.5 MPa [14]. Finally, Shaffer used glass fiber fabric in ionic liquid-based epoxy matrices as structural separators in structural supercapacitors that exhibited a shear modulus of 895 MPa and a shear strength of 8.71 MPa [15].
Multifunctional Efficiency
A notable result from prior work was the emergence of a multifunctional efficiency metric (equations 1–3) for assessing the structural energy materials’ ability to reduce mass without sacrificing energy storage capabilities [1]. This is described by the multifunctional efficiency, ηmf , which is the sum of the energy and structural contributions, ηe and ηs respectively:
where Γ , E , and UTS are the specific mf mf mf energy, specific Young’s modulus, and specific ultimate tensile strength, respectively, of the multifunctional material. Similarly, Γ , E , and UTS are the specific energy, specific Young’s modulus, and specific ultimate tensile strength, respectively, of a traditional energy or structural material. When η is mf greater than unity, the system is considered to deliver energy at a mass savings [1]. The Lutkenhaus group proposed an alternative selection criterion that allows the user to weight the structural energy and power material according to a specific project’s need. This is described as the utility (U), equations 4–6 [16]:
where ECU is electrochemical utility, MU is mechanical utility, and a is a weighting coefficient that varies from 0 to 1 (0 prioritizes electrochemical performance and 1 prioritizes mechanical properties). Cmf is specific capacitance or capacity at different scan rates or current densities ν, σmf is the ultimate tensile strength, Emf is the Young’s modulus, ε mf is the ultimate strain, and Tmf is the toughness of the multifunctional material. C, σ, E, ε, and T are the capacitance or capacity, strength, Young’s modulus, ultimate strain, and toughness, respectively, of a traditional energy or structural materials.
The benefit of this approach is that it can be tailored to specific applications using the weighting coefficient a. For example, a multifunctional material can be designed to withstand significant mechanical stresses while providing a small amount of energy storage for replacing vehicle paneling or support by biasing a toward 1. This metric also accounts for rate capability and several other mechanical properties simultaneously. The utility equation can be easily modified to add or remove terms to suit the user’s needs. However, while utility can compare the ability of materials to effectively deliver energy and act as a structural support, it cannot supply a condition that would indicate mass-savings as with ηmf [1]. Another option is to use a multifunctional metric that is multiplicative rather than summative [17].
Composite Systems
One potential method to fabricate structural electrodes with greater energy storage capabilities is to use composite systems. Aramid nanofibers (ANFs), a recently discovered nanoscale building block derived from Kevlar fibers, are an ideal filler candidate for mechanical reinforcement [18]. Kevlar fibers are popular for their use in bulletproof vests because of Kevlar’s high Young’s modulus and tensile strength of 129 GPa and 4.1 GPa, respectively [19].
In addition, ANFs are easily fabricated through the dissolution of Kevlar in dimethyl sulfoxide and potassium hydroxide via deprotonation of the amide groups. These desirable properties have led to a surge in the production of mechanically robust composite materials using ANFs, making them a natural choice for structural energy materials.
Our team has focused on developing structural supercapacitors electrodes based on reduced graphene oxide (rGO) and ANF composites using experiments [6, 20, 21] and computation [16, 22]. rGO nanosheets are related to graphene, a single-layered sheet of sp2-hybridized carbon. Oxidizing the parent material, graphite, into graphene oxide with various oxygen-containing functional groups (epoxy, hydroxyl, and carboxyl groups) disrupts the sp2 -hybridization and allows for easier processing of the sheets— which are otherwise prone to agglomeration.
Graphene oxide nanosheets may be reduced using chemical, thermal, or electrochemical means to partially restore the sp2 -hybridization and yield rGO nanosheets [23]. rGO was chosen due to its excellent electrical properties, promising mechanical properties, and ease of processing [24, 25]. Also, rGO nanosheets are capable of noncovalent interactions, such as hydrogen bonding and π-π stacking, which lead to enhanced mechanical properties for the rGO/ ANF composite electrode.
RGO/ANF Structural Electrodes
Vacuum-assisted self-assembly of rGO and ANF dispersions was used to fabricate the composite, free-standing electrodes with a brick-and-mortar morphology (see Figure 2a) [20]. The incorporation of ANFs led to a dramatic increase in the mechanical properties. Tensile stress-strain curves in Figure 2b show that Young’s modulus increased by ~350% (from 3.7 GPa to 13.0 GPa) and tensile strength increased by ~290% (from 34.4 MPa to 100.6 MPa) when 25 wt% ANF was added to rGO. This massive increase was attributed to the noncovalent interactions between the rGO sheets and the ANFs. As for the supercapacitor performance, small amounts of ANFs slightly improved the rate capability of the electrodes due to promoting rGO sheet separation, leading to more facile ion diffusion. However, the specific capacitance of the electrode decreased with the introduction of ANF, which is electrochemically inactive. Cyclic voltammetry curves are displayed in Figure 2c. Despite this decrease in capacitance, the rGO/ANF electrodes possessed an excellent ηmf of 3.2 (modulus-based) and 1.5 (strength-based) for 25 wt% ANF electrodes when compared against carbon aerogels and epoxy.
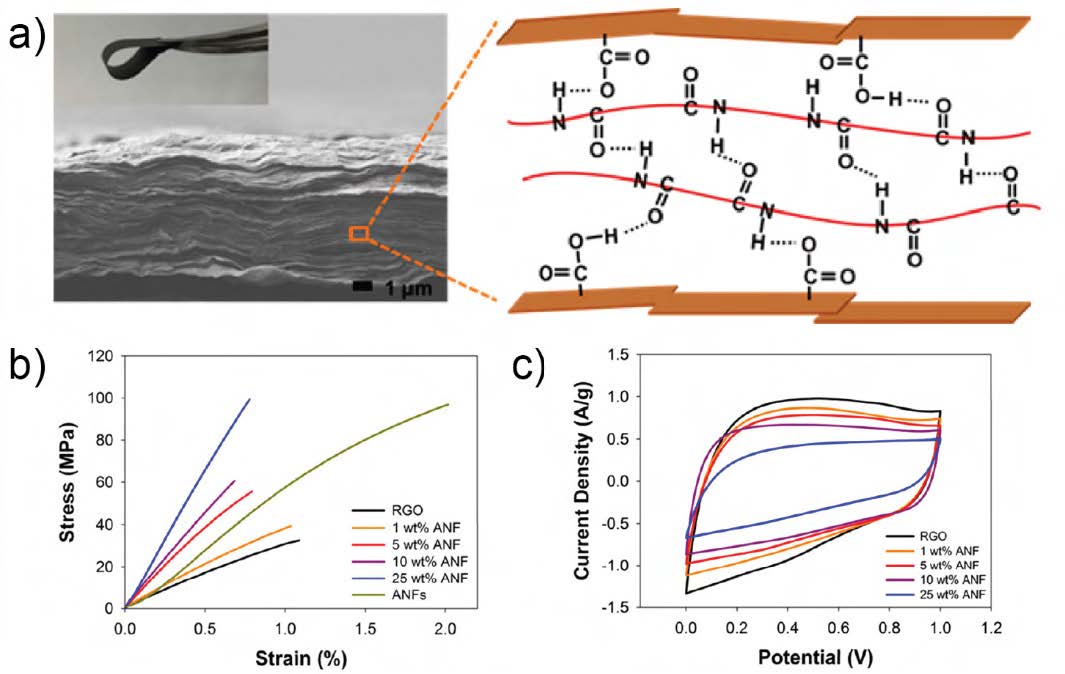
Figure 2. a) Cross-sectional SEM image of rGO/ANF electrode with inset image shows electrode under bending and schematic of brick-and-mortar morphology and interaction of rGO sheets and ANFs through hydrogen bonding. b) Stress-strain curves and c) cyclic voltammetry curvesfor rGO/ANF composites [20]. Reproduced with permission. Copyright 2017 American Chemical Society.
Micromechanics Modelling
Along with experimental investigations, computational methods were also used to study rGO/ANF electrodes. Using the Mori-Tanaka method, Boyd and Lagoudas developed a model to capture the effects of rGO nanosheet and ANF waviness on the mechanical properties of the overall composite [22]. Waviness describes the conformation of the nanomaterials, as it is known that they are not perfectly flat or straight. The flowchart for the modelling depicted in Figure 3 shows how the nanomaterials were modelled individually and then combined into a real volume element that described the composite. This model identified the significant influence of rGO and ANF waviness on the elastic modulus of the composite electrode, with increased waviness resulting in lower modulus. Accordingly, the rGO nanosheets and ANFs should be as flat or straight as possible to maximize the mechanical properties.
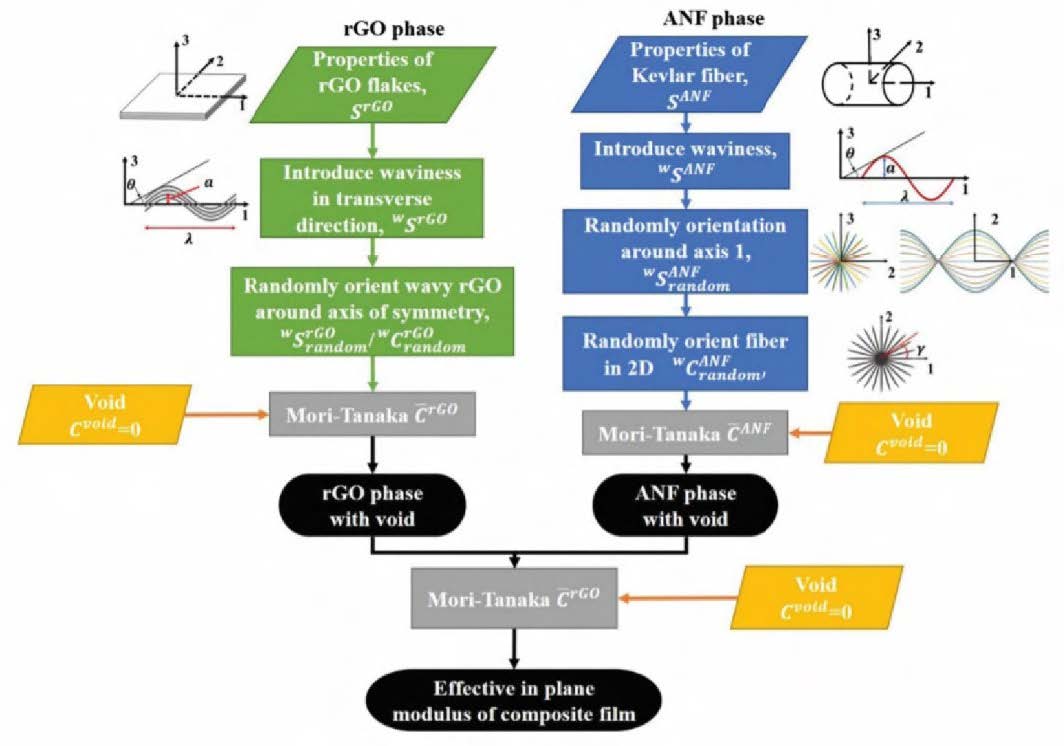
Figure 3. Flowchart for micro-mechanical modeling of rGO/ANF electrodes [22]
Interfacial Engineering
Recognizing that interfacial interactions between the rGO nanosheets and ANFs play a major role in the success of previously developed structural electrodes, the Lutkenhaus group explored enhancing these interactions through functionalization of the rGO nanosheets with carboxylic acid (-COOH) or amine (-NH2 ) groups [6]. The functionalized rGO nanosheets were then combined with ANFs using vacuum-assisted self-assembly to obtain electrodes. The Young’s modulus and strength increased because of the additional hydrogen bonding provided by the -COOH and -NH2 groups, with the -NH2 functionalization showing the greatest enhancement. Specifically, the Young’s modulus increased by 18% (from 9.9 GPa to 12.2 GPa), and the strength increased by 24% (from 79 MPa to 98 MPa) for 25 wt% ANF electrodes containing -NH2 functionalized rGO nanosheets. However, the electrochemical performance decreased with functionalization due to the introduction of defects in the sp2 carbon network. Fortunately, the functionalized rGO/ANF electrodes showed an excellent combination of mechanical and
electrochemical properties as compared to similar materials (see Figure 4a). The electrodes exhibited a high ηmf , indicating potential mass-savings for replacing steel and epoxy (see Figure 4b)
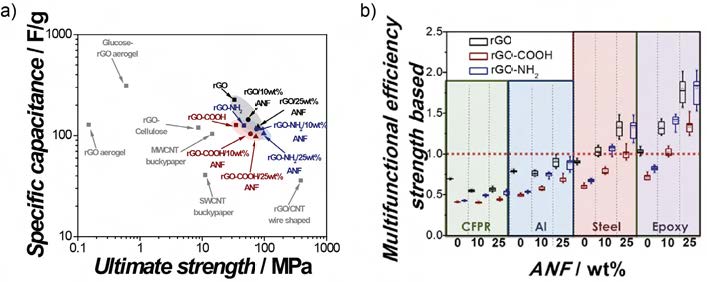
Figure 4. a) Ashby plot comparing specific capacitance versus ultimate strength for various electrodes and rGO/ANF composites. b) ηmf (strength-based) based on carbon aerogels and either carbon fiber reinforced epoxy (green), aluminum (blue), steel (red), and epoxy (purple) [6]
Materials Informatics Analysis
Data science was also applied to this work using materials informatics analysis [16]. As opposed to traditional computational modelling, materials informatics does not use physics-based modelling, but instead uses previous data to predict properties and performance. The benefit of materials informatics is that it reduces the number of experiments and cost required to identify a global optimum in structural energy and power materials performance.
Lutkenhaus and Arróyave specifically examined rGO, ANF, and carbon nanotube (CNT) composite electrodes. CNTs were added to improve electronic conductivity between rGO nanosheets [26]. Materials informatics was used to guide experimental design toward an optimal combination of the three components by suggesting the next experiment to execute. After performing the suggested experiment, the new data were added to the model to obtain a new suggested experimental condition. Using this data science approach, an optimal composition (with a 78.8% increase to Young’s modulus and a 34.0% increase to strength relative to the initial data set) was identified in only six iterations.
Research Summary
Summarizing the work on rGO/ANF structural energy and power electrodes for supercapacitors, it is generally shown that these electrodes exhibited excellent ηmf . The rGO/ ANF composite electrodes exhibited a higher capacitance but lower modulus as compared to carbon fibers. Chemical functionalization of the rGO nanosheets improved the mechanical properties by providing non-covalent interactions with the ANFs. However, functionalization yielded drops in electrochemical performance due to defects in the graphene’s sp2 carbon network. Further, addition of ANFs might enhance mechanical properties, but ANFs ultimately dilute the electrochemically active components. Accordingly, computational modeling was applied to understand further routes to improve the performance by physics-based modeling of the waviness of the nanomaterials or by data science to determine an optimal electrode composition.
Future Research and Potential DoD Applications
Despite significant strides toward viable structural energy and power materials, the ultimate challenge still remains: finding the right balance amid the trade-off between mechanical and electrochemical performance. To meet this challenge, comprehensive physics-based models that holistically capture mechanoelectrochemical response are needed. Also, the complex interactions between energy storage and mechanical stress must be deduced using in situ mechanoelectrochemical testing. Finally, the role of the interphase and the interactions between rGO nanosheets and ANFs should be elucidated so that interphase might be engineered without reduction in electrochemical performance.
Components that act as both batteries and structural panels hold great promise for high-value DoD applications. For example, DoD could incorporate this technology into ground and aerial vehicles to increase power storage without increasing mass or volume of the vehicle, or to increase operational range. This would allow additional energy for onboard devices or for propulsion in electric vehicles. The technology could also be used to reduce the mass and volume of the vehicle while maintaining energy storage, resulting in higher fuel efficiency and smaller footprints. This is particularly pertinent in applications where mass or volume are highly valued, such as satellites, unmanned aerial vehicles, and powered protective vests for the warfighter [27].
References
1. O’Brien, D. J., Baechle, D. M., & Wetzel, E. D. (2011). Design and performance of multifunctional structural composite capacitors. Journal of Composite Materials, 45(26), 2797–2809. doi:10.1177/0021998311412207
2. Snyder, J., Gienger, E., & Wetzel, E. (2015). Performance metrics for structural composites with electrochemical multifunctionality. Journal of Composite Materials, 49(15), 1835– 1848. doi:10.1177/0021998314568167
3. Asp, L. E., & Greenhalgh, E. S. (2014). Structural power composites. Composites Science and Technology, 101, 41–61. doi:10.1016/j.compscitech.2014.06.020
4. Snyer, J. F., Carter, R. H., Xu, K., Wong, E. I., Nguyen, P. A., Ho, E. H., & Wetzel, E. D. (2007, September). Multifunctional Structural Composite Batteries for U.S. Army Applications. U.S. Army Research Laboratory. Retrieved from https://apps. dtic.mil/dtic/tr/fulltext/u2/a472034.pdf
5. Tadjeh, Y. (2018, May 22). SOCOM pursuing “Hyper-Enabled Operator” technologies. National Defense. Re- trieved from http://www.nationaldefensemagazine.org/articles/2018/5/22/ socom-pursuing-hyper-enabled-operator-technologies
6. Flouda, P., Feng, X., Boyd, J. G., Thomas, E. L., Lagoudas, D. C., & Lutkenhaus, J. L. (2019). Interfacial Engineering of Reduced Graphene Oxide for Aramid Nanofiber-Enabled Structural Supercapacitors. Batteries & Supercaps, 0(0). doi:10.1002/batt.201800137
7. Zhang, X., Sui, Z., Xu, B., Yue, S., Luo, Y., Zhan, W., & Liu, B. (2011). Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. Journal of Materials Chemistry, 21(18), 6494–6497. doi:10.1039/ C1JM10239G
8. Gurevitch, I., Buonsanti, R., Teran, A. A., Gludovatz, B., Ritchie, R. O., Cabana, J., & Balsara, N. P. (2013). Nanocomposites of titanium ioxide and polystyrene-poly(ethylene oxide) block copolymer as solid-state electrolytes for lithium metal batteries. Journal of The Electrochemical Society, 160(9), A1611–A1617. doi:10.1149/2.117309jes
9. Sheidaei, A., Xiao, X., Huang, X., & Hitt, J. (2011). Mechanical behavior of a battery separator in electrolyte solutions. Journal of Power Sources, 196(20), 8728–8734. doi:10.1016/j.jpowsour.2011.06.026
10.Muralidharan, N., Teblum, E., Westover, A. S., Schauben, D., Itzhak, A., Muallem, M., . . . Pint, C. L. (2018). Carbon nanotube reinforced structural composite supercapacitor. Scientific Reports, 8(1), 17662. doi:10.1038/s41598-018- 34963-x
11. Carlson, T., Ordéus, D., Wysocki, M., & Asp, L. E. (2010). Structural capacitor materials made from carbon fibre epoxy composites. Composites Science and Technology, 70(7), 1135–1140. doi:10.1016/j.compscitech.2010.02.028
12.Ye, Y., Xiao, H., Reaves, K., McCulloch, B., Mike, J. F., & Lutkenhaus, J. L. (2018). Effect of nanorod aspect ratio on shear thickening electrolytes for safety-enhanced batteries. ACS Applied Nano Materials, 1(6), 2774–2784. doi:10.1021/acsanm.8b00457
13.Veith, G. M., Armstrong, B. L., Wang, H., Kalnaus, S., Tenhaeff, W. E., & Patterson, M. L. (2017). Shear thickening electrolytes for high impact resistant batteries. ACS Energy Letters, 2(9), 2084–2088. doi:10.1021/acsenergylett.7b00511
14.Yao, P., Zhu, B., Zhai, H., Liao, X., Zhu, Y., Xu, W., . . . Yang, Y. (2018). PVDF/Palygorskite nanowire composite electrolyte for 4 V rechargeable lithium batteries with high energy density. Nano Letters, 18(10), 6113–6120. doi:10.1021/acs.nanolett.8b01421
15.Qian, H., Kucernak, A. R., Greenhalgh, E. S., Bismarck, A., & Shaffer, M. S. P. (2013). Multifunctional structural supercapacitor composites based on carbon aerogel modified high performance carbon fiber fabric. ACS Applied Materials & Interfaces, 5(13), 6113–6122. doi:10.1021/am400947j
16.Patel, A., Johnson, L., Arroyave, R., & Lutkenhaus, J. (2019). Design of multifunctional supercapacitor electrodes using an informatics approach. Molecular Systems Design & Engineering. doi:10.1039/C8ME00060C
17.Sun, W., Shah, S. A., Lowery, J. A., Oh, J. H., Lutkenhaus, J. L., & Green, M. J. (2019). Lightweight Kevlar® reinforced graphene oxide architectures with high strength for energy storage. Submitted.
18.Yang, M., Cao, K., Sui, L., Qi, Y., Zhu, J., Waas, A., . . . Kotov, N. A. (2011). Dispersions of aramid nanofibers: A new nanoscale building block. ACS Nano, 5(9), 6945–6954. doi:10.1021/ nn2014003
19.Young, R. J., Lu, D., Day, R. J., Knoff, W. F., & Davis, H. A. (1992). Relationship between structure and mechanical properties for aramid fibres. Journal of Materials Science, 27(20), 5431–5440. doi:10.1007/bf00541602
20.Kwon, S. R., Harris, J., Zhou, T., Loufakis, D., Boyd, J. G., & Lutkenhaus, J. L. (2017). Mechanically strong graphene/ aramid nanofiber composite electrodes for structural energy and power. ACS Nano, 11(7), 6682–6690. doi:10.1021/ acsnano.7b00790
21.Kwon, S. R., Elinski, M. B., Batteas, J. D., & Lutkenhaus, J. L. (2017). Robust and flexible aramid nanofiber/graphene layer-by-layer electrodes. ACS Applied Materials & Interfaces, 9(20), 17125– 17135. doi:10.1021/acsami.7b03449
22.Zhou, T., Boyd, J. G., Lutkenhaus, J. L., & Lagoudas, D. C. (2019). Micromechanics modeling of the elastic moduli of rGO/ANF nanocomposites. Acta Mechanica, 230(1), 265–280. doi:10.1007/ s00707-018-2298-9
23.Dreyer, D. R., Park, S., Bielawski, C. W., & Ruoff, R. S. (2010). The chemistry of graphene oxide. Chemical Society Reviews, 39(1), 228–240. doi:10.1039/ B917103G
24.Park, S., & Ruoff, R. S. (2009). Chemical methods for the production of graphenes. Nature Nanotechnology, 4, 217. doi:10.1038/nnano.2009.58
25.Stoller, M. D., Park, S., Zhu, Y., An, J., & Ruoff, R. S. (2008). Graphene-based ultracapacitors. Nano Letters, 8(10), 3498–3502. doi:10.1021/nl802558y
26.Pham, D. T., Lee, T. H., Luong, D. H., Yao, F., Ghosh, A., Le, V. T., . . . Lee, Y. H. (2015). Carbon nanotube-bridged graphene 3D building blocks for ultrafast compact supercapacitors. ACS Nano, 9(2), 2018–2027. doi:10.1021/ nn507079x
27.Iriarte, M. (2019, April 15). Military UAVs tackle performance issues under SWaP-driven designs. Military Embedded Systems. Retrieved from http://mil-embedded.com/articles/ military-uavs-tackle-performance-issues-under-swap-driven-designs/


